Vascular cell signaling by membrane estrogen receptors
- PMID: 18325557
- PMCID: PMC2519041
- DOI: 10.1016/j.steroids.2008.01.008
Vascular cell signaling by membrane estrogen receptors
Abstract
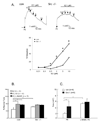
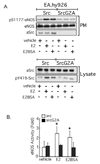
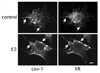
The definition of estrogen's actions has expanded from transcriptional regulation to the rapid, membrane-initiated activation of numerous signal transduction cascades. Multiple biological effects of estrogen have been shown in numerous animals, cellular and molecular studies, which support the favorable effects of estrogen on vascular structure, function, and cell signaling. Work from several laboratories has shown that these effects are mediated by distinct forms of estrogen receptor (ER) alpha. This includes estrogen-stimulated rapid activation of endothelial nitric oxide synthase (eNOS), resulting in the elaboration of the athero-protective, angiogenesis-promoting product nitric oxide (NO). We have described the expression of ER46, an N-terminus truncated isoform of the ERalpha, in human endothelial cells (EC), and its critical role in membrane-initiated, rapid responses to 17beta-estradiol (E2). We have proposed an ER46-centered, eNOS activating molecular complex in human EC caveolar membranes, containing c-Src, phosphatidylinositol 3-kinase (PI3K), Akt and eNOS. Our previous studies support estrogen-induced rapid eNOS activation via a sequential c-Src/PI3K/Akt cascade in EC. In this review, we describe estrogen-induced, rapid, non-genomic actions in endothelium, driven by c-Src-ER46-caveolin-1 interactions, with consequent activation of eNOS. Amidst ongoing controversies in hormone replacement therapy, these molecular and cellular data, defining favorable estrogenic effects on the endothelium, provide a strong impetus to resolve these clinical questions.
Figures


Similar articles
-
Membrane-initiated actions of estrogen on the endothelium.Mol Cell Endocrinol. 2009 Sep 24;308(1-2):3-8. doi: 10.1016/j.mce.2009.03.025. Epub 2009 Apr 9. Mol Cell Endocrinol. 2009. PMID: 19549586 Free PMC article. Review.
-
Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen.J Biol Chem. 2003 Jan 24;278(4):2118-23. doi: 10.1074/jbc.M210828200. Epub 2002 Nov 12. J Biol Chem. 2003. PMID: 12431978
-
Endothelial estrogen receptor isoforms and cardiovascular disease.Mol Cell Endocrinol. 2014 May 25;389(1-2):65-70. doi: 10.1016/j.mce.2014.02.001. Epub 2014 Feb 11. Mol Cell Endocrinol. 2014. PMID: 24530925 Free PMC article. Review.
-
Vascular cell signaling by membrane estrogen receptors.Steroids. 2005 May-Jun;70(5-7):382-7. doi: 10.1016/j.steroids.2005.02.011. Epub 2005 Mar 16. Steroids. 2005. PMID: 15862821 Review.
-
Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells.Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4807-12. doi: 10.1073/pnas.0831079100. Epub 2003 Apr 7. Proc Natl Acad Sci U S A. 2003. PMID: 12682286 Free PMC article.
Cited by
-
17ß-estradiol antagonizes the down-regulation of ERα/NOS-3 signaling in vascular endothelial dysfunction of female diabetic rats.PLoS One. 2012;7(11):e50402. doi: 10.1371/journal.pone.0050402. Epub 2012 Nov 29. PLoS One. 2012. PMID: 23209733 Free PMC article.
-
Therapy for Pulmonary Arterial Hypertension: Glance on Nitric Oxide Pathway.Front Pharmacol. 2021 Nov 12;12:767002. doi: 10.3389/fphar.2021.767002. eCollection 2021. Front Pharmacol. 2021. PMID: 34867394 Free PMC article. Review.
-
Dehydroabietic acid isolated from Commiphora opobalsamum causes endothelium-dependent relaxation of pulmonary artery via PI3K/Akt-eNOS signaling pathway.Molecules. 2014 Jun 23;19(6):8503-17. doi: 10.3390/molecules19068503. Molecules. 2014. PMID: 24959678 Free PMC article.
-
Role of GPER in estrogen-dependent nitric oxide formation and vasodilation.J Steroid Biochem Mol Biol. 2018 Feb;176:65-72. doi: 10.1016/j.jsbmb.2017.05.006. Epub 2017 May 18. J Steroid Biochem Mol Biol. 2018. PMID: 28529128 Free PMC article.
-
Efficacy of Local N-Acetylcysteine Administration in Mitigating OHSS Parameters: A Comparative Analysis With Dopaminergic Agonist in the OHSS Model.Int J Endocrinol. 2024 Dec 5;2024:1634072. doi: 10.1155/ije/1634072. eCollection 2024. Int J Endocrinol. 2024. PMID: 39669379 Free PMC article.
References
-
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. - PubMed
-
- Stamper MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20:47–63. - PubMed
-
- Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–1037. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

