Microglial responses to dopamine in a cell culture model of Parkinson's disease
- PMID: 18325635
- PMCID: PMC2762863
- DOI: 10.1016/j.neurobiolaging.2008.01.001
Microglial responses to dopamine in a cell culture model of Parkinson's disease
Abstract
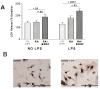
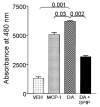
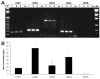
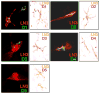
Activated microglia appear to selectively attack dopamine (DA) neurons in the Parkinson's disease (PD) substantia nigra. We investigated potential mechanisms using culture models. As targets, human SH-SY5Y cells were left undifferentiated (UNDIFF) or were differentiated with retinoic acid (RA) or RA plus brain-derived neurotrophic factor (RA/BDNF). RA/BDNF-treated cells were immunoreactive for tyrosine hydroxylase and the DA transporter, took up exogenous DA, and released DA after K(+) stimulation. Undifferentiated and RA-treated cells lacked these characteristics of a DA phenotype. Co-culture of target cells with human elderly microglia resulted in elevated toxicity in DA phenotype (RA/BDNF) cells. Lipopolysaccharide (LPS) plus K(+)-stimulated DA release enhanced toxicity by 500-fold. DA induced microglial chemotaxis in Boyden chambers. Spiperone inhibited this effect. Cultured human elderly microglia expressed mRNAs for D1-D4 but not D5 DA receptors. The microglia, as well as PD microglia in situ, were also immunoreactive for D1-D4 but not D5 DA receptors. These findings demonstrate that activated microglia express DA receptors, and suggest that this mechanism may play a role in the selective vulnerability of DA neurons in PD.
Conflict of interest statement
There are no actual or potential conflicts of interest that the authors have.
Figures





References
-
- Akiyama H, McGeer PL. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 1989;489:247–253. - PubMed
-
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimunol. 1990;30:81–93. - PubMed
-
- Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis. 2003;12:35–45. - PubMed
-
- Chang RC, Hudson PM, Wilson BC, Liu B, Abel H, Hong JS. High concentrations of extracellular postassium enhance bacterial endotoxin lipopolysaccharide-induced neurotoxicity in glia-neuron mixed cultures. Neuroscience. 2000;97:757–764. - PubMed
-
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willet WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

