Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k
- PMID: 18325990
- PMCID: PMC2408805
- DOI: 10.1210/en.2007-1655
Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k
Abstract
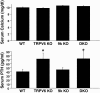
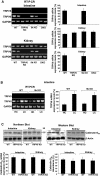
To study the role of the epithelial calcium channel transient receptor potential vanilloid type 6 (TRPV6) and the calcium-binding protein calbindin-D9k in intestinal calcium absorption, TRPV6 knockout (KO), calbindin-D9k KO, and TRPV6/calbindin-D(9k) double-KO (DKO) mice were generated. TRPV6 KO, calbindin-D9k KO, and TRPV6/calbindin-D9k DKO mice have serum calcium levels similar to those of wild-type (WT) mice ( approximately 10 mg Ca2+/dl). In the TRPV6 KO and the DKO mice, however, there is a 1.8-fold increase in serum PTH levels (P < 0.05 compared with WT). Active intestinal calcium transport was measured using the everted gut sac method. Under low dietary calcium conditions there was a 4.1-, 2.9-, and 3.9-fold increase in calcium transport in the duodenum of WT, TRPV6 KO, and calbindin-D9k KO mice, respectively (n = 8-22 per group; P > 0.1, WT vs. calbindin-D9k KO, and P < 0.05, WT vs. TRPV6 KO on the low-calcium diet). Duodenal calcium transport was increased 2.1-fold in the TRPV6/calbindin-D9k DKO mice fed the low-calcium diet (P < 0.05, WT vs. DKO). Active calcium transport was not stimulated by low dietary calcium in the ileum of the WT or KO mice. 1,25-Dihydroxyvitamin D3 administration to vitamin D-deficient null mutant and WT mice also resulted in a significant increase in duodenal calcium transport (1.4- to 2.0-fold, P < 0.05 compared with vitamin D-deficient mice). This study provides evidence for the first time using null mutant mice that significant active intestinal calcium transport occurs in the absence of TRPV6 and calbindin-D9k, thus challenging the dogma that TRPV6 and calbindin-D9k are essential for vitamin D-induced active intestinal calcium transport.
Figures

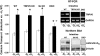
References
-
- Christakos S, Dhawan P, Liu Y, Peng X, Porta A 2003 New insights into the mechanisms of vitamin D action. J Cell Biochem 88:695–705 - PubMed
-
- Wasserman RH, Fullmer CS 1995 Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr 125:1971S–1979S - PubMed
-
- Raval-Pandya M, Porta AR, Christakos S 1998 Mechanism of action of 1,25 dihydroxyvitamin D3 on intestinal calcium absorption and renal calcium transportation. In: Holick MF, ed. Vitamin D: physiology, molecular biology, and clinical applications. Totowa, NJ: Humana Press; 163–174
-
- Feher JJ 1983 Facilitated calcium diffusion by intestinal calcium-binding protein. Am J Physiol Cell Physiol 244:C303–C307 - PubMed
-
- Bronner F, Pansu D, Stein WD 1986 An analysis of intestinal calcium transport across the rat intestine. Am J Physiol 250:G561–G569 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

