Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes
- PMID: 18326497
- PMCID: PMC2442320
- DOI: 10.1074/jbc.M707697200
Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes
Abstract
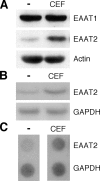
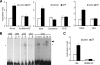
Glutamate is an essential neurotransmitter regulating brain functions. Excitatory amino acid transporter (EAAT)-2 is one of the major glutamate transporters primarily expressed in astroglial cells. Dysfunction of EAAT2 is implicated in acute and chronic neurological disorders, including stroke/ischemia, temporal lobe epilepsy, amyotrophic lateral sclerosis, Alzheimer disease, human immunodeficiency virus 1-associated dementia, and growth of malignant gliomas. Ceftriaxone, one of the beta-lactam antibiotics, is a stimulator of EAAT2 expression with neuroprotective effects in both in vitro and in vivo models based in part on its ability to inhibit neuronal cell death by glutamate excitotoxicity. Based on this consideration and its lack of toxicity, ceftriaxone has potential to manipulate glutamate transmission and ameliorate neurotoxicity. We investigated the mechanism by which ceftriaxone enhances EAAT2 expression in primary human fetal astrocytes (PHFA). Ceftriaxone elevated EAAT2 transcription in PHFA through the nuclear factor-kappaB (NF-kappaB) signaling pathway. The antibiotic promoted nuclear translocation of p65 and activation of NF-kappaB. The specific NF-kappaB binding site at the -272 position of the EAAT2 promoter was responsible for ceftriaxone-mediated EAAT2 induction. In addition, ceftriaxone increased glutamate uptake, a primary function of EAAT2, and EAAT2 small interference RNA completely inhibited ceftriaxone-induced glutamate uptake activity in PHFA. Taken together, our data indicate that ceftriaxone is a potent modulator of glutamate transport in PHFA through NF-kappaB-mediated EAAT2 promoter activation. These findings suggest a mechanism for ceftriaxone modulation of glutamate transport and for its potential effects on ameliorating specific neurodegenerative diseases through modulation of extracellular glutamate.
Figures



References
-
- Fonnum, F. (1984) J. Neurochem. 42 1-11 - PubMed
-
- Headley, P. M., and Grillner, S. (1990) Trends Pharmacol. Sci. 11 205-211 - PubMed
-
- Anderson, C. M., and Swanson, R. A. (2000) Glia 32 1-14 - PubMed
-
- Rothstein, J. D., Martin, L., Levey, A. I., Dykes-Hoberg, M., Jin, L., Wu, D., Nash, N., and Kuncl, R. W. (1994) Neuron 13 713-725 - PubMed
-
- Plaitakis, A., and Caroscio, J. T. (1987) Ann. Neurol. 22 575-579 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

