Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial
- PMID: 18326503
- PMCID: PMC2267989
- DOI: 10.1136/bmj.39503.582396.25
Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial
Abstract
Objective: To evaluate the impact of telling patients their estimated spirometric lung age as an incentive to quit smoking.
Design: Randomised controlled trial.
Setting: Five general practices in Hertfordshire, England.
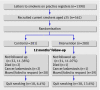
Participants: 561 current smokers aged over 35.
Intervention: All participants were offered spirometric assessment of lung function. Participants in intervention group received their results in terms of "lung age" (the age of the average healthy individual who would perform similar to them on spirometry). Those in the control group received a raw figure for forced expiratory volume at one second (FEV1). Both groups were advised to quit and offered referral to local NHS smoking cessation services.
Main outcome measures: The primary outcome measure was verified cessation of smoking by salivary cotinine testing 12 months after recruitment. Secondary outcomes were reported changes in daily consumption of cigarettes and identification of new diagnoses of chronic obstructive lung disease.
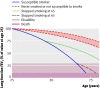
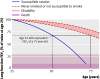
Results: Follow-up was 89%. Independently verified quit rates at 12 months in the intervention and control groups, respectively, were 13.6% and 6.4% (difference 7.2%, P=0.005, 95% confidence interval 2.2% to 12.1%; number needed to treat 14). People with worse spirometric lung age were no more likely to have quit than those with normal lung age in either group. Cost per successful quitter was estimated at 280 pounds sterling (366 euros, $556). A new diagnosis of obstructive lung disease was made in 17% in the intervention group and 14% in the control group; a total of 16% (89/561) of participants.
Conclusion: Telling smokers their lung age significantly improves the likelihood of them quitting smoking, but the mechanism by which this intervention achieves its effect is unclear.
Trial registration: National Research Register N0096173751.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Incentives to quit smoking in primary care.BMJ. 2008 Mar 15;336(7644):567-8. doi: 10.1136/bmj.39506.386759.80. Epub 2008 Mar 6. BMJ. 2008. PMID: 18326502 Free PMC article.
-
Lung age: Study's conclusion about screening is unwarranted.BMJ. 2008 May 10;336(7652):1034. doi: 10.1136/bmj.39556.492176.80. BMJ. 2008. PMID: 18467391 Free PMC article. No abstract available.
-
Telling smokers their "lung age" promoted successful smoking cessation.Evid Based Nurs. 2008 Jul;11(3):76. doi: 10.1136/ebn.11.3.76. Evid Based Nurs. 2008. PMID: 18583488 No abstract available.
-
Telling smokers their "lung age" promoted successful smoking cessation.ACP J Club. 2008 Jul;149(1):5. ACP J Club. 2008. PMID: 18624373 No abstract available.
-
Telling smokers their "lung age" promoted successful smoking cessation.Evid Based Med. 2008 Aug;13(4):104. doi: 10.1136/ebm.13.4.104. Evid Based Med. 2008. PMID: 18667663 No abstract available.
-
Help smokers quit: tell them their "lung age".J Fam Pract. 2008 Sep;57(9):584-6. J Fam Pract. 2008. PMID: 18786330 Free PMC article.
References
-
- Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Prev Med 1985;14:655-62. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical