Role of small GTPase Rho in regulating corneal epithelial wound healing
- PMID: 18326710
- PMCID: PMC2661132
- DOI: 10.1167/iovs.07-1122
Role of small GTPase Rho in regulating corneal epithelial wound healing
Abstract
Purpose: To determine the role of small GTPase Rho and its relation with epidermal growth factor receptor (EGFR) in mediating corneal epithelial wound healing.
Methods: Rho activity in THCE cells, an SV40-immortalized human corneal epithelial cell (HCEC) line, and primary HCECs was assessed by pull-down assay followed by Western blotting. Rho functions were inhibited with specific inhibitor exoenzyme C3 (C3) and confirmed by knockdown with small interference RNA (siRNA) transfection. Effects of Rho inhibition on wound healing were determined in porcine corneal organ culture and HCEC scratch wound models. Effects of C3 on cell proliferation and focal adhesion formation were determined by BrdU incorporation assay and immunocytochemistry, respectively.
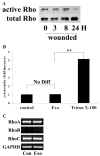
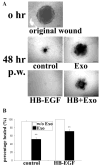
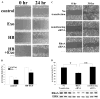
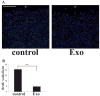
Results: Wounding, lysophosphatidic acid, and heparin-binding EGF-like growth factor (HB-EGF) induced rapid and strong RhoA activation. HB-EGF-, but not wounding-, enhanced RhoA activity was sensitive to EGFR inhibition. In corneal organ and cell culture models, C3 attenuated spontaneous and HB-EGF-induced wound closures, confirmed by delayed wound healing in cells transfected with RhoA siRNA. C3 also retarded spontaneous wound healing in the presence of hydroxyurea, a cell cycle blocker. C3 significantly reduced the number of BrdU-positive cells near the leading edge. Treatment with C3 resulted in the disruption of the cortical actin cytoskeleton and in the disappearance of paxillin-containing focal adhesion and lamellipodia.
Conclusions: Wounding induces RhoA activation through an EGFR-independent pathway. Rho activity is required for modulating cell migration and proliferation through cytoskeleton reorganization and focal adhesion formation in response to wounding.
Figures




Similar articles
-
Rho kinases regulate corneal epithelial wound healing.Am J Physiol Cell Physiol. 2008 Aug;295(2):C378-87. doi: 10.1152/ajpcell.90624.2007. Epub 2008 May 21. Am J Physiol Cell Physiol. 2008. PMID: 18495812 Free PMC article.
-
Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor.Invest Ophthalmol Vis Sci. 2007 Feb;48(2):636-43. doi: 10.1167/iovs.06-0203. Invest Ophthalmol Vis Sci. 2007. PMID: 17251460 Free PMC article.
-
Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells.Invest Ophthalmol Vis Sci. 2004 Mar;45(3):813-20. doi: 10.1167/iovs.03-0851. Invest Ophthalmol Vis Sci. 2004. PMID: 14985295 Free PMC article.
-
Growth factors and corneal epithelial wound healing.Brain Res Bull. 2010 Feb 15;81(2-3):229-35. doi: 10.1016/j.brainresbull.2009.08.024. Epub 2009 Sep 4. Brain Res Bull. 2010. PMID: 19733636 Free PMC article. Review.
-
Roles of P21-activated kinases and associated proteins in epithelial wound healing.Int Rev Cell Mol Biol. 2008;267:253-98. doi: 10.1016/S1937-6448(08)00606-0. Int Rev Cell Mol Biol. 2008. PMID: 18544501 Free PMC article. Review.
Cited by
-
Therapeutic Potential of Rho Kinase Inhibitors in Corneal Disease: A Systematic Review of Preclinical and Clinical Studies.Biomedicines. 2025 Jun 30;13(7):1602. doi: 10.3390/biomedicines13071602. Biomedicines. 2025. PMID: 40722674 Free PMC article. Review.
-
RHO-Associated Coiled-Coil-Containing Protein Kinase Inhibitors Significantly Modulate the Epithelial-Mesenchymal Transition Induced by TGF-β2 in the 2-D and 3-D Cultures of Human Corneal Stroma Fibroblasts.Biomedicines. 2024 Dec 6;12(12):2784. doi: 10.3390/biomedicines12122784. Biomedicines. 2024. PMID: 39767691 Free PMC article.
-
Progress in corneal wound healing.Prog Retin Eye Res. 2015 Nov;49:17-45. doi: 10.1016/j.preteyeres.2015.07.002. Epub 2015 Jul 18. Prog Retin Eye Res. 2015. PMID: 26197361 Free PMC article. Review.
-
In vitro transdifferentiation of corneal epithelial-like cells from human skin-derived precursor cells.Int J Ophthalmol. 2012;5(2):158-63. doi: 10.3980/j.issn.2222-3959.2012.02.08. Epub 2012 Apr 18. Int J Ophthalmol. 2012. PMID: 22762041 Free PMC article.
-
Low levels of hydrogen peroxide stimulate corneal epithelial cell adhesion, migration, and wound healing.Invest Ophthalmol Vis Sci. 2011 Mar 25;52(3):1723-34. doi: 10.1167/iovs.10-5866. Print 2011 Mar. Invest Ophthalmol Vis Sci. 2011. PMID: 21087961 Free PMC article.
References
-
- Agrawal VB, Tsai RJ. Corneal epithelial wound healing. Indian J Ophthalmol. 2003;51(1):5–15. - PubMed
-
- Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. 2001;226(7):653–664. - PubMed
-
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. - PubMed
-
- Zieske JD. Extracellular matrix and wound healing. Curr Opin Ophthalmol. 2001;12(4):237–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

