Development and dynamics of robust T-cell responses to CML under imatinib treatment
- PMID: 18326818
- PMCID: PMC2396727
- DOI: 10.1182/blood-2007-12-128397
Development and dynamics of robust T-cell responses to CML under imatinib treatment
Abstract
Novel molecular targeted therapies, such as imatinib for chronic myelogenous leukemia (CML), represent the first agents that inhibit cancer cells more than other dividing cells, such as immune cells. We hypothesize that imatinib may create a window in which the immune response is partially restored while apoptotic leukemic cells are present, thus rendering leukemic cells immunogenic as patients enter remission. To detect and quantify antileukemia immune responses in an antigen-unbiased way, we used cryopreserved autologous pretreatment blood samples (representing predominantly leukemic cells) as stimulators to detect antileukemia T-cell responses in CML patients in remission on imatinib. We studied patients over time to address the dynamics of such responses. Our data show that antileukemia T-cell responses develop in the majority of CML patients (9 of 14) in remission and that CD4(+) T cells producing tumor necrosis factor-alpha (median 17.6%) represent the major response over interferon-gamma. This confirms the immune system's ability to respond to leukemia under certain conditions. Such responses may be further amplified as a potential therapy that synergizes with imatinib for improved control of CML.
Figures
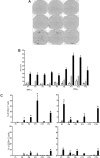
 ). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone (
). The following stimulated remission samples from the patients are shown and depicted as median and range: P1, 5 months; P2, 12 months; P3, 6 months; P4, 18 months; P6, 14 months; P8, 15 months; P11, 4 months; P12, 5 months; P13, 12 months. All responses were statistically significant (P < .05) compared with leukemic or remission samples alone. (C) TNF-α and IFN-γ production (CFC) by CD4+ T and CD8+ T cells in stimulated remission samples (■) from 6 patients. Leukemic cells alone (□) and remission samples alone ( ). The same remission samples shown in panel B were analyzed (*P < .05, statistically significant responses over background).
). The same remission samples shown in panel B were analyzed (*P < .05, statistically significant responses over background).

 ). Background levels are connected by lines (*P < .05, statistically significant responses over background). Ratio of the tumor load measured by molecular responses (bcr-abl transcript/bcr-abl control gene, left panel, ○) and levels of Ph+ cells in chromosome analysis (right panel, ▵) at the corresponding time points. (B) TNF-α (■) and IFN-γ(▾) responses by CD4+ and CD8+ T cells (CFC) from patients P4 and P13 over time. Leukemic cells alone (TNF-α (■), IFN-γ(▾)) and remission samples alone (TNF-α (
). Background levels are connected by lines (*P < .05, statistically significant responses over background). Ratio of the tumor load measured by molecular responses (bcr-abl transcript/bcr-abl control gene, left panel, ○) and levels of Ph+ cells in chromosome analysis (right panel, ▵) at the corresponding time points. (B) TNF-α (■) and IFN-γ(▾) responses by CD4+ and CD8+ T cells (CFC) from patients P4 and P13 over time. Leukemic cells alone (TNF-α (■), IFN-γ(▾)) and remission samples alone (TNF-α ( ), IFN-γ(
), IFN-γ( ). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.
). Background levels are connected by lines (*P < .05, statistically significant responses over background). (C) Tumor load over time from patients P5(▴), P6(▾), P7 (♦), P8 (●), connected by solid lines, and patients P9 (□), P11 (▿), and P14 (○), connected by dotted lines. Error bars indicate ranges of triplicate measurements. Results are representative of 2 or 3 independent experiments.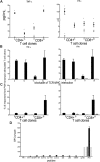
 ) and T-cell clones stimulated with remission sample (
) and T-cell clones stimulated with remission sample ( ). (B,C) Responses in T-cell clones stimulated with remission sample pulsed with lysate from autologous leukemic cells (■) and remission sample pulsed with lysate from the AML cell line THP1 (
). (B,C) Responses in T-cell clones stimulated with remission sample pulsed with lysate from autologous leukemic cells (■) and remission sample pulsed with lysate from the AML cell line THP1 ( ) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.
) using CFC for TNF-α and IFN-γ (B) and blocked with anti-CD4 and anti-MHC II antibodies or anti-CD8 and anti-MHC II antibodies, respectively (C). Results were compared with PHA and ionomycin-stimulated T-cell clones. (D) T-cell responses in CD8+ T-cell clones pulsed with HLA-A0201 restricted peptides from LAAs and TAAs using ELISPOT assay compared with PHA stimulated CD8+ T-cell clones, and CD8+ T-cell clones stimulated with a peptide from the melanoma-associated antigen gp100. Results are shown as median and range.
 ), and leukemic cells alone (□). Results are shown as median and range. Immune responses in prestimulated remission samples using CFC: (B) 28 months; (C) 32 months. Remission samples from P10 stimulated with autologous leukemic cells (third column), unstimulated remission samples (second column), and leukemic cells alone (first columns). (D) Tumor load from patient P10 over time (○).
), and leukemic cells alone (□). Results are shown as median and range. Immune responses in prestimulated remission samples using CFC: (B) 28 months; (C) 32 months. Remission samples from P10 stimulated with autologous leukemic cells (third column), unstimulated remission samples (second column), and leukemic cells alone (first columns). (D) Tumor load from patient P10 over time (○).References
-
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. - PubMed
-
- Lee SJ. Chronic myelogenous leukaemia. Br J Haematol. 2000;111:993–1009. - PubMed
-
- Angstreich GR, Smith BD, Jones RJ. Treatment options for chronic myeloid leukemia: imatinib versus interferon versus allogeneic transplant. Curr Opin Oncol. 2004;16:95–99. - PubMed
-
- Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

