Endogenous morphine in SH-SY5Y cells and the mouse cerebellum
- PMID: 18327293
- PMCID: PMC2265639
- DOI: 10.1371/journal.pone.0001641
Endogenous morphine in SH-SY5Y cells and the mouse cerebellum
Abstract
Background: Morphine, the principal active agent in opium, is not restricted to plants, but is also present in different animal tissues and cell types, including the mammalian brain. In fact, its biosynthetic pathway has been elucidated in a human neural cell line. These data suggest a role for morphine in brain physiology (e.g., neurotransmission), but this hypothesis remains a matter of debate. Recently, using the adrenal neuroendocrine chromaffin cell model, we have shown the presence of morphine-6-glucuronide (M6G) in secretory granules and their secretion products, leading us to propose that these endogenous alkaloids might represent new neuroendocrine factors. Here, we investigate the potential function of endogenous alkaloids in the central nervous system.
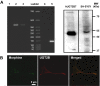
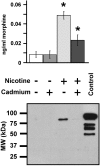
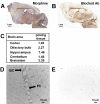
Methodology and principal findings: Microscopy, molecular biology, electrophysiology, and proteomic tools were applied to human neuroblastoma SH-SY5Y cells (i) to characterize morphine and M6G, and (ii) to demonstrate the presence of the UDP-glucuronyltransferase 2B7 enzyme, which is responsible for the formation of M6G from morphine. We show that morphine is secreted in response to nicotine stimulation via a Ca(2+)-dependent mechanism involving specific storage and release mechanisms. We also show that morphine and M6G at concentrations as low as 10(-10) M are able to evoke specific naloxone-reversible membrane currents, indicating possible autocrine/paracrine regulation in SH-SY5Y cells. Microscopy and proteomic approaches were employed to detect and quantify endogenous morphine in the mouse brain. Morphine is present in the hippocampus, cortex, olfactory bulb, and cerebellum at concentration ranging from 1.45 to 7.5 pmol/g. In the cerebellum, morphine immunoreactivity is localized to GABA basket cells and their termini, which form close contacts on Purkinje cell bodies.
Conclusions/significance: The presence of morphine in the brain and its localization in particular areas lead us to conclude that it has a specific function in neuromodulation and/or neurotransmission. Furthermore, its presence in cerebellar basket cell termini suggests that morphine has signaling functions in Purkinje cells that remain to be discovered.
Conflict of interest statement
Figures




References
-
- Stefano GB, Goumon Y, Casares F, Cadet P, Fricchione GL, et al. Endogenous morphine. Trends Neurosci. 2000;23:436–442. - PubMed
-
- Hazum E, Sabatka JJ, Chang KJ, Brent DA, Findlay JW, et al. Morphine in cow and human milk: could dietary morphine constitute a ligand for specific morphine (mu) receptors? Science. 1981;213:1010–1012. - PubMed
-
- Gintzler AR, Gershon MD, Spector S. A nonpeptide morphine-like compound: immunocytochemical localization in the mouse brain. Science. 1978;199:447–448. - PubMed
-
- Herbert RB, Venter H, Pos S. Do mammals make their own morphine? Nat Prod Rep. 2000;17:317–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

