Opioid detoxification via single 7-day application of a buprenorphine transdermal patch: an open-label evaluation
- PMID: 18327673
- PMCID: PMC7351294
- DOI: 10.1007/s00213-008-1105-z
Opioid detoxification via single 7-day application of a buprenorphine transdermal patch: an open-label evaluation
Abstract
Rationale: Managed withdrawal (i.e., detoxification) from opioid dependence is a widespread clinical procedure that is a necessary step for those pursuing abstinence. Buprenorphine is one effective detoxification treatment, however, consensus regarding effective detoxification procedures is lacking.
Objectives: This study evaluated the efficacy of a buprenorphine transdermal formulation (i.e., patch) in suppressing opioid withdrawal, its safety and tolerability, and its biodelivery when applied for 7 days.
Methods: Physically dependent opioid (heroin) users (n = 12) completed a 10-day opioid detoxification in a residential research unit. Each received a single patch application that remained in place for 7 days. Blood samples were drawn prior to patch application and once daily thereafter. Assessments, four times daily, included: the amount of rescue medications ordered to treat withdrawal discomfort; self-report and observer ratings of opioid withdrawal and agonist effects; and vital sign measures.
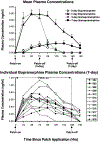
Results: Overall, the patch appeared safe and well-tolerated. Buprenorphine plasma levels peaked 48 h after patch application at 0.59 ng/ml. Indices of withdrawal (self-reports, observer ratings, rescue medication) were significantly reduced within 24 h of patch application, continued to decline thereafter, and did not reappear following patch removal.
Conclusions: This study confirms that transdermal buprenorphine is safe and clinically effective, and suggests that a 7-day application may provide an effective and comfortable means of detoxification. This patch formulation would appear to be a useful opioid detoxification treatment by reducing compliance concerns, and administering buprenorphine in a formulation less likely to be diverted to illicit use.
Conflict of interest statement
Figures


References
-
- Amass L, Bickel WK, Higgins ST, Hughes JR (1994) A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J Addict Dis 13:33–45 - PubMed
-
- Amass L, Ling W, Freese TE, Reiber C, Annon JJ, Cohen AJ, McCarty D, Reid MS, Brown LS, Clark C, Ziedonis DM, Krejci J, Stine S, Winhusen T, Brigham G, Babcock D, Muir JA, Buchan BJ, Horton T (2004) Bringing buprenorphine-naloxone detoxification to community treatment providers: the NIDA Clinical Trials Network field experience. Am J Addict 13(Suppl 1):S42–S66 - PMC - PubMed
-
- Amato L, Davoli M, Ferri M, Gowing L, Perucci CA (2004) Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug Alcohol Depend 73:219–226 - PubMed
-
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE (1988a) A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther 43:72–78 - PubMed
-
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE (1988b) Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther 247:47–53 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

