HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion
- PMID: 18328425
- PMCID: PMC2643426
- DOI: 10.1016/j.ccr.2008.01.034
HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion
Abstract
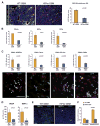
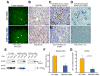
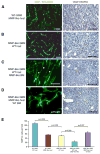
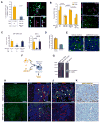
Development of hypoxic regions is an indicator of poor prognosis in many tumors. Here, we demonstrate that HIF1alpha, the direct effector of hypoxia, partly through increases in SDF1alpha, induces recruitment of bone marrow-derived CD45+ myeloid cells containing Tie2+, VEGFR1+, CD11b+, and F4/80+ subpopulations, as well as endothelial and pericyte progenitor cells to promote neovascularization in glioblastoma. MMP-9 activity of bone marrow-derived CD45+ cells is essential and sufficient to initiate angiogenesis by increasing VEGF bioavailability. In the absence of HIF1alpha, SDF1alpha levels decrease, and fewer BM-derived cells are recruited to the tumors, decreasing MMP-9 and mobilization of VEGF. VEGF also directly regulates tumor cell invasiveness. When VEGF activity is impaired, tumor cells invade deep into the brain in the perivascular compartment.
Figures


Comment in
-
A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization.Cancer Cell. 2008 Mar;13(3):181-3. doi: 10.1016/j.ccr.2008.02.016. Cancer Cell. 2008. PMID: 18328420 Free PMC article.
References
-
- Aghi M, Chiocca EA. Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol Ther. 2005;12:994–1005. - PubMed
-
- Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. - PubMed
-
- Behrendtsen O, Werb Z. Metalloproteinases regulate parietal endoderm differentiating and migrating in cultured mouse embryos. Dev Dyn. 1997;208:255–265. - PubMed
-
- Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

