Current and future antiviral therapy of severe seasonal and avian influenza
- PMID: 18328578
- PMCID: PMC2346583
- DOI: 10.1016/j.antiviral.2008.01.003
Current and future antiviral therapy of severe seasonal and avian influenza
Abstract
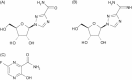
The currently circulating H3N2 and H1N1 subtypes of influenza A virus cause a transient, febrile upper respiratory illness in most adults and children ("seasonal influenza"), but infants, the elderly, immunodeficient and chronically ill persons may develop life-threatening primary viral pneumonia or complications such as bacterial pneumonia. By contrast, avian influenza viruses such as the H5N1 virus that recently emerged in Southeast Asia can cause severe disease when transferred from domestic poultry to previously healthy people ("avian influenza"). Most H5N1 patients present with fever, cough and shortness of breath that progress rapidly to adult respiratory distress syndrome. In seasonal influenza, viral replication remains confined to the respiratory tract, but limited studies indicate that H5N1 infections are characterized by systemic viral dissemination, high cytokine levels and multiorgan failure. Gastrointestinal infection and encephalitis also occur. The licensed anti-influenza drugs (the M2 ion channel blockers, amantadine and rimantadine, and the neuraminidase inhibitors, oseltamivir and zanamivir) are beneficial for uncomplicated seasonal influenza, but appropriate dosing regimens for severe seasonal or H5N1 viral infections have not been defined. Treatment options may be limited by the rapid emergence of drug-resistant viruses. Ribavirin has also been used to a limited extent to treat influenza. This article reviews licensed drugs and treatments under development, including high-dose oseltamivir; parenterally administered neuraminidase inhibitors, peramivir and zanamivir; dimeric forms of zanamivir; the RNA polymerase inhibitor T-705; a ribavirin prodrug, viramidine; polyvalent and monoclonal antibodies; and combination therapies.
Figures



References
-
- Apisarnthanarak A., Kitphati R., Thongphubeth K., Patoomanunt P., Anthanont P., Auwanit W., Thawatsupha P., Chittaganpitch M., Saeng-Aroon S., Waicharoen S., Apisarnthanarak P., Storch G.A., Mundy L.M., Fraser V.J. Atypical avian influenza (H5N1) Emerg. Infect. Dis. 2004;10:1321–1324. - PMC - PubMed
-
- Arabi Y., Gommersall C., Ahmed Q., Boynton B., Memish Z. The critically ill avian influenza A (H5N1) patient. Crit. Care Med. 2007;35:1397–1403. - PubMed
-
- Babu Y.S., Chand P., Bantia S., Kotian P., Dehghani A., El-Kattan Y., Lin T.H., Hutchison T.L., Elliott A.J., Parker C.D., Ananth S.L., Horn L.L., Laver G.W., Montgomery J.A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 2000;43:3482–3486. - PubMed
-
- Bantia S., Arnold C.S., Parker C.D., Upshaw R., Chand P. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antivir. Res. 2006;69:39–45. - PubMed
-
- Beare A.S., Webster R.G. Replication of avian influenza viruses in humans. Arch. Virol. 1991;119:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

