Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses
- PMID: 18328829
- PMCID: PMC2888475
- DOI: 10.1016/j.bbcan.2008.02.001
Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses
Abstract
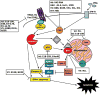
Tremendous advances have been made in developing oncolytic viruses (OVs) in the last few years. By taking advantage of current knowledge in cancer biology and virology, specific OVs have been genetically engineered to target specific molecules or signal transduction pathways in cancer cells in order to achieve efficient and selective replication. The viral infection and amplification eventually induce cancer cells into cell death pathways and elicit host antitumor immune responses to further help eliminate cancer cells. Specifically targeted molecules or signaling pathways (such as RB/E2F/p16, p53, IFN, PKR, EGFR, Ras, Wnt, anti-apoptosis or hypoxia) in cancer cells or tumor microenvironment have been studied and dissected with a variety of OVs such as adenovirus, herpes simplex virus, poxvirus, vesicular stomatitis virus, measles virus, Newcastle disease virus, influenza virus and reovirus, setting the molecular basis for further improvements in the near future. Another exciting new area of research has been the harnessing of naturally tumor-homing cells as carrier cells (or cellular vehicles) to deliver OVs to tumors. The trafficking of these tumor-homing cells (stem cells, immune cells and cancer cells), which support proliferation of the viruses, is mediated by specific chemokines and cell adhesion molecules and we are just beginning to understand the roles of these molecules. Finally, we will highlight some avenues deserving further study in order to achieve the ultimate goals of utilizing various OVs for effective cancer treatment.
Figures


References
-
- Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. - PubMed
-
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. - PubMed
-
- Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525–538. - PubMed
-
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. - PubMed
-
- Xia ZJ, Chang JH, Zhang L, Jiang WQ, Guan ZZ, Liu JW, Zhang Y, Hu XH, Wu GH, Wang HQ, Chen ZC, Chen JC, Zhou QH, Lu JW, Fan QX, Huang JJ, Zheng X. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng. 2004;23:1666–1670. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

