Brain microglia express steroid-converting enzymes in the mouse
- PMID: 18329265
- PMCID: PMC2423427
- DOI: 10.1016/j.jsbmb.2007.12.013
Brain microglia express steroid-converting enzymes in the mouse
Abstract
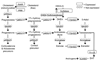
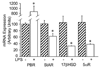
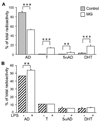
In the CNS, steroid hormones play a major role in the maintenance of brain homeostasis and it's response to injury. Since activated microglia are the pivotal immune cell involved in neurodegeneration, we investigated the possibility that microglia provide a discrete source for the metabolism of active steroid hormones. Using RT-PCR, our results showed that mouse microglia expressed mRNA for 17beta-hydroxysteroid dehydrogenase type 1 and steroid 5alpha-reductase type 1, which are involved in the metabolism of androgens and estrogens. Microglia also expressed the peripheral benzodiazepine receptor and steroid acute regulatory protein; however, the enzymes required for de novo formation of progesterone and DHEA from cholesterol were not expressed. To test the function of these enzymes, primary microglia cultures were incubated with steroid precursors, DHEA and AD. Microglia preferentially produced delta-5 androgens (Adiol) from DHEA and 5alpha-reduced androgens from AD. Adiol behaved as an effective estrogen receptor agonist in neuronal cells. Activation of microglia with pro-inflammatory factors, LPS and INFgamma did not affect the enzymatic properties of these proteins. However, PBR ligands reduced TNFalpha production signifying an immunomodulatory role for PBR. Collectively, our results suggest that microglia utilize steroid-converting enzymes and related proteins to influence inflammation and neurodegeneration within microenvironments of the brain.
Figures





Similar articles
-
Androgen formation and metabolism in the pulmonary epithelial cell line A549: expression of 17beta-hydroxysteroid dehydrogenase type 5 and 3alpha-hydroxysteroid dehydrogenase type 3.Endocrinology. 2000 Aug;141(8):2786-94. doi: 10.1210/endo.141.8.7589. Endocrinology. 2000. PMID: 10919264
-
Differential androgen and estrogen substrates specificity in the mouse and primates type 12 17beta-hydroxysteroid dehydrogenase.J Endocrinol. 2007 Aug;194(2):449-55. doi: 10.1677/JOE-07-0144. J Endocrinol. 2007. PMID: 17641292
-
Expression levels of mRNA for neurosteroidogenic enzymes 17β-HSD, 5α-reductase, 3α-HSD and cytochrome P450 aromatase in the fetal wild type and SF-1 knockout mouse brain.Endocr Res. 2015;40(1):44-8. doi: 10.3109/07435800.2014.933974. Epub 2014 Aug 11. Endocr Res. 2015. PMID: 25111584
-
DHEA and peripheral androgen and estrogen formation: intracinology.Ann N Y Acad Sci. 1995 Dec 29;774:16-28. doi: 10.1111/j.1749-6632.1995.tb17369.x. Ann N Y Acad Sci. 1995. PMID: 8597456 Review. No abstract available.
-
Pathways and genes involved in steroid hormone metabolism in male pigs: a review and update.J Steroid Biochem Mol Biol. 2014 Mar;140:44-55. doi: 10.1016/j.jsbmb.2013.11.001. Epub 2013 Nov 12. J Steroid Biochem Mol Biol. 2014. PMID: 24239507 Review.
Cited by
-
Microglial Cell Morphology and Phagocytic Activity Are Critically Regulated by the Neurosteroid Allopregnanolone: A Possible Role in Neuroprotection.Cells. 2021 Mar 21;10(3):698. doi: 10.3390/cells10030698. Cells. 2021. PMID: 33801063 Free PMC article.
-
Human Microglia Synthesize Neurosteroids to Cope with Rotenone-Induced Oxidative Stress.Antioxidants (Basel). 2023 Apr 19;12(4):963. doi: 10.3390/antiox12040963. Antioxidants (Basel). 2023. PMID: 37107338 Free PMC article.
-
Sex-specific responses of the pubertal neuroimmune axis in CD-1 mice.Brain Behav Immun Health. 2021 Feb 24;13:100229. doi: 10.1016/j.bbih.2021.100229. eCollection 2021 May. Brain Behav Immun Health. 2021. PMID: 34589744 Free PMC article.
-
Sex and hormonal influences on seizures and epilepsy.Horm Behav. 2013 Feb;63(2):267-77. doi: 10.1016/j.yhbeh.2012.03.018. Epub 2012 Apr 4. Horm Behav. 2013. PMID: 22504305 Free PMC article. Review.
-
BV-2 Microglial Cells Respond to Rotenone Toxic Insult by Modifying Pregnenolone, 5α-Dihydroprogesterone and Pregnanolone Levels.Cells. 2020 Sep 13;9(9):2091. doi: 10.3390/cells9092091. Cells. 2020. PMID: 32933155 Free PMC article.
References
-
- Bryant DN, et al. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29(2):199–207. - PubMed
-
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. - PubMed
-
- Garcia-Ovejero D, et al. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48(2):273–286. - PubMed
-
- Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101(4):1252–1261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

