Localization of A20 to a lysosome-associated compartment and its role in NFkappaB signaling
- PMID: 18329387
- PMCID: PMC2587335
- DOI: 10.1016/j.bbamcr.2008.01.029
Localization of A20 to a lysosome-associated compartment and its role in NFkappaB signaling
Abstract
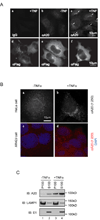
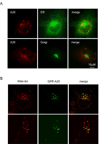
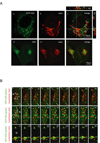
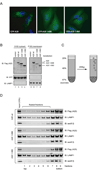
A20 is a tumor necrosis factor (TNF)-inducible zinc finger protein that contains both ubiquitinating and deubiquitinating activities. A20 negatively regulates NFkappaB (nuclear factor kappaB) signaling induced by TNF receptor family and Toll-like receptors, but the mechanism of A20 action is poorly defined. Here we show that a fraction of endogenous and ectopically expressed A20 is localized to an endocytic membrane compartment that is in association with the lysosome. The lysosomal association of A20 requires its carboxy terminal zinc finger domains, but is independent of its ubiquitin-modifying activities. Interestingly, A20 mutants defective in membrane association also contain reduced NFkappaB inhibitory activity. These findings suggest the involvement of a lysosome-associated mechanism in A20-dependent termination of NFkappaB signaling.
Figures

References
-
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. - PubMed
-
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. - PubMed
-
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. - PubMed
-
- Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. - PubMed
-
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

