Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry
- PMID: 18329684
- PMCID: PMC2519004
- DOI: 10.1016/j.virol.2008.01.044
Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry
Abstract
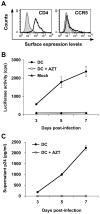
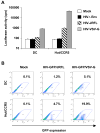
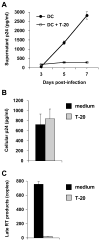
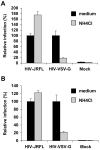
Human immunodeficiency virus type 1 (HIV-1) enters dendritic cells (DCs) through endocytosis and viral receptor-mediated fusion. Although endocytosis-mediated HIV-1 entry can generate productive infection in certain cell types, including human monocyte-derived macrophages, productive HIV-1 infection in DCs appears to be dependent on fusion-mediated viral entry. It remains to be defined whether endocytosed HIV-1 in DCs can initiate productive infection. Using HIV-1 infection and cellular fractionation assays to measure productive viral infection and entry, here we show that HIV-1 enters monocyte-derived DCs predominately through endocytosis; however, endocytosed HIV-1 cannot initiate productive HIV-1 infection in DCs. In contrast, productive HIV-1 infection in DCs requires fusion-mediated viral entry. Together, these results provide functional evidence in understanding HIV-1 cis-infection of DCs, suggesting that different pathways of HIV-1 entry into DCs determine the outcome of viral infection.
Figures


References
-
- Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier JL, Arenzana-Seisdedos F, Amara A. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol. 2006;80(6):2949–2957. - PMC - PubMed
-
- Cavrois M, De Noronha C, Greene WC. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol. 2002;20(11):1151–1154. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

