Chronic CXCL10 alters the level of activated ERK1/2 and transcriptional factors CREB and NF-kappaB in hippocampal neuronal cell culture
- PMID: 18329727
- PMCID: PMC2396565
- DOI: 10.1016/j.jneuroim.2008.01.003
Chronic CXCL10 alters the level of activated ERK1/2 and transcriptional factors CREB and NF-kappaB in hippocampal neuronal cell culture
Abstract
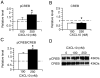
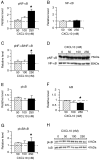
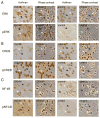
Signal transduction pathways may be important targets of chemokines during neuroinflammation. In the current study, Western blot analyses show that in rat hippocampal neuronal/glial cell cultures chronic CXCL10 increases the level of protein for ERK1/2 as well as for the transcriptional factors CREB and NF-kappaB. Bcl-2, an anti-apoptotic protein whose expression can be regulated by a pathway involving ERK1/2, CREB and NF-kappaB, was also increased in the CXCL10 treated cultures. These results implicate a role for ERK1/2, CREB and NF-kappaB in effects of CXCL10 on hippocampal cells and suggest that chronic CXCL10 may have a protective role during certain neuroinflammatory conditions.
Figures


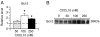
References
-
- Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. - PubMed
-
- Bahr BA, Bendiske J, Brown QB, Munirathinam S, Caba E, Rudin M, Urwyler S, Sauter A, Rogers G. Survival signaling and selective neuroprotection through glutamatergic transmission. Exp Neurol. 2002;174:37–47. - PubMed
-
- Bailey CH, Kaang BK, Chen M, Martin KC, Lim CS, Casadio A, Kandel ER. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron. 1997;18:913–924. - PubMed
-
- Biber K, Sauter A, Brouwer N, Copray SC, Boddeke HW. Ischemia-induced neuronal expression of the microglia attracting chemokine Secondary Lymphoid-tissue Chemokine (SLC) Glia. 2001;34:121–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

