On helicases and other motor proteins
- PMID: 18329872
- PMCID: PMC2396192
- DOI: 10.1016/j.sbi.2008.01.007
On helicases and other motor proteins
Abstract
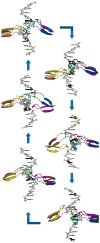
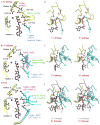
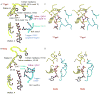
Helicases are molecular machines that utilize energy derived from ATP hydrolysis to move along nucleic acids and to separate base-paired nucleotides. The movement of the helicase can also be described as a stationary helicase that pumps nucleic acid. Recent structural data for the hexameric E1 helicase of papillomavirus in complex with single-stranded DNA and MgADP has provided a detailed atomic and mechanistic picture of its ATP-driven DNA translocation. The structural and mechanistic features of this helicase are compared with the hexameric helicase prototypes T7gp4 and SV40 T-antigen. The ATP-binding site architectures of these proteins are structurally similar to the sites of other prototypical ATP-driven motors such as F1-ATPase, suggesting related roles for the individual site residues in the ATPase activity.
Figures

References
-
- Ilyina TV, Gorbalenya AE, Koonin EV. Organization and evolution of bacterial and bacteriophage primase-helicase systems. J Mol Evol. 1992;34:351–357. - PubMed
-
- Gorbalenya AE, Koonin EV. Helicases: amino acid sequence comparisons and structure-function relationships. Current Opinion in Structural Biology. 1993;3:419–429. This manuscript outlines multiple conserved sequence motifs that define two helicase superfamilies.
-
- Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem. 2007;76:23–50. This recent review article details the structure and mechanism of multiple superfamilies of helicases, defining precise details within the superfamilies and noting common features between them. - PubMed
-
- Mastrangelo IA, Hough PV, Wall JS, Dodson M, Dean FB, Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989;338:658–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

