Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions
- PMID: 18332111
- PMCID: PMC2423145
- DOI: 10.1128/MCB.00144-08
Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions
Abstract
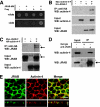
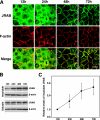
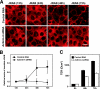
Tight junctions (TJs) are cell-cell adhesive structures that undergo continuous remodeling. We previously demonstrated that Rab13 and a junctional Rab13-binding protein (JRAB)/molecule interacting with CasL-like 2 (MICAL-L2) localized at TJs and mediated the endocytic recycling of the integral TJ protein occludin and the formation of functional TJs. Here, we investigated how JRAB/MICAL-L2 was targeted to TJs. Using a series of deletion mutants, we found the plasma membrane (PM)-targeting domain within JRAB/MICAL-L2. We then identified actinin-4, which was originally isolated as an actin-binding protein associated with cell motility and cancer invasion/metastasis, as a binding protein for the PM-targeting domain of JRAB/MICAL-L2, using a yeast two-hybrid system. Actinin-4 was colocalized with JRAB/MICAL-L2 at cell-cell junctions and linked JRAB/MICAL-L2 to F-actin. Although actinin-4 bound to JRAB/MICAL-L2 without Rab13, the actinin-4-JRAB/MICAL-L2 interaction was enhanced by Rab13 activation. Depletion of actinin-4 by using small interfering RNA inhibited the recruitment of occludin to TJs during the Ca(2+) switch. During the epithelial polarization after replating, JRAB/MICAL-L2 was recruited from the cytosol to cell-cell junctions. This JRAB/MICAL-L2 recruitment as well as the formation of functional TJs was delayed in actinin-4-depleted cells. These results indicate that actinin-4 is involved in recruiting JRAB/MICAL-L2 to cell-cell junctions and forming functional TJs.
Figures





Similar articles
-
The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions.Mol Biol Cell. 2008 Mar;19(3):971-83. doi: 10.1091/mbc.e07-06-0551. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094055 Free PMC article.
-
JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin.Mol Biol Cell. 2006 May;17(5):2465-75. doi: 10.1091/mbc.e05-09-0826. Epub 2006 Mar 8. Mol Biol Cell. 2006. PMID: 16525024 Free PMC article.
-
Identification and characterization of JRAB/MICAL-L2, a junctional Rab13-binding protein.Methods Enzymol. 2008;438:141-53. doi: 10.1016/S0076-6879(07)38010-5. Methods Enzymol. 2008. PMID: 18413246
-
Rab family small G proteins in regulation of epithelial apical junctions.Front Biosci (Landmark Ed). 2009 Jan 1;14(6):2115-29. doi: 10.2741/3366. Front Biosci (Landmark Ed). 2009. PMID: 19273188 Review.
-
Regulation of epithelial cell adhesion and repulsion: role of endocytic recycling.J Med Invest. 2008 Feb;55(1-2):9-16. doi: 10.2152/jmi.55.9. J Med Invest. 2008. PMID: 18319540 Review.
Cited by
-
Connecdenn 3/DENND1C binds actin linking Rab35 activation to the actin cytoskeleton.Mol Biol Cell. 2012 Jan;23(1):163-75. doi: 10.1091/mbc.E11-05-0474. Epub 2011 Nov 9. Mol Biol Cell. 2012. PMID: 22072793 Free PMC article.
-
α-Actinin-4 is required for amoeboid-type invasiveness of melanoma cells.J Biol Chem. 2014 Nov 21;289(47):32717-28. doi: 10.1074/jbc.M114.579185. Epub 2014 Oct 8. J Biol Chem. 2014. PMID: 25296750 Free PMC article.
-
Diverse functions for the semaphorin receptor PlexinD1 in development and disease.Dev Biol. 2011 Jan 1;349(1):1-19. doi: 10.1016/j.ydbio.2010.09.008. Epub 2010 Sep 27. Dev Biol. 2011. PMID: 20880496 Free PMC article. Review.
-
MICAL-L2 Is Essential for c-Myc Deubiquitination and Stability in Non-small Cell Lung Cancer Cells.Front Cell Dev Biol. 2021 Jan 14;8:575903. doi: 10.3389/fcell.2020.575903. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33520979 Free PMC article.
-
MICAL-family proteins: Complex regulators of the actin cytoskeleton.Antioxid Redox Signal. 2014 May 1;20(13):2059-73. doi: 10.1089/ars.2013.5487. Epub 2013 Aug 17. Antioxid Redox Signal. 2014. PMID: 23834433 Free PMC article. Review.
References
-
- Anderson, J., C. Van Itallie, and A. Fanning. 2004. Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 16140-145. - PubMed
-
- Ando-Akatsuka, Y., S. Yonemura, M. Itoh, M. Furuse, and S. Tsukita. 1999. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell Physiol. 179115-125. - PubMed
-
- Bertet, C., L. Sulak, and T. Lecuit. 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429667-671. - PubMed
-
- Braga, V., and A. Yap. 2005. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr. Opin. Cell Biol. 17466-474. - PubMed
-
- Broderick, M., and S. Winder. 2005. Spectrin, α-actinin, and dystrophin. Adv. Protein Chem. 70203-246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
