Subunit a facilitates aqueous access to a membrane-embedded region of subunit c in Escherichia coli F1F0 ATP synthase
- PMID: 18332132
- PMCID: PMC2431001
- DOI: 10.1074/jbc.M800901200
Subunit a facilitates aqueous access to a membrane-embedded region of subunit c in Escherichia coli F1F0 ATP synthase
Abstract
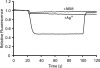
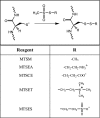
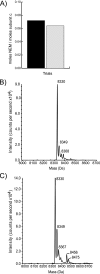
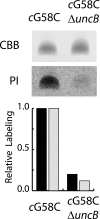
Rotary catalysis in F(1)F(0) ATP synthase is powered by proton translocation through the membrane-embedded F(0) sector. Proton binding and release occurs in the middle of the membrane at Asp-61 on transmembrane helix 2 of subunit c. Previously, the reactivity of cysteines substituted into F(0) subunit a revealed two regions of aqueous access, one extending from the periplasm to the middle of the membrane and a second extending from the middle of the membrane to the cytoplasm. To further characterize aqueous accessibility at the subunit a-c interface, we have substituted Cys for residues on the cytoplasmic side of transmembrane helix 2 of subunit c and probed the accessibility to these substituted positions using thiolate-reactive reagents. The Cys substitutions tested were uniformly inhibited by Ag(+) treatment, which suggested widespread aqueous access to this generally hydrophobic region. Sensitivity to N-ethylmaleimide (NEM) and methanethiosulfonate reagents was localized to a membrane-embedded pocket surrounding Asp-61. The cG58C substitution was profoundly inhibited by all the reagents tested, including membrane impermeant methanethiosulfonate reagents. Further studies of the highly reactive cG58C substitution revealed that NEM modification of a single c subunit in the oligomeric c-ring was sufficient to cause complete inhibition. In addition, NEM modification of subunit c was dependent upon the presence of subunit a. The results described here provide further evidence for an aqueous-accessible region at the interface of subunits a and c extending from the middle of the membrane to the cytoplasm.
Figures

Similar articles
-
Aqueous accessibility to the transmembrane regions of subunit c of the Escherichia coli F1F0 ATP synthase.J Biol Chem. 2009 Aug 28;284(35):23243-50. doi: 10.1074/jbc.M109.002501. Epub 2009 Jun 19. J Biol Chem. 2009. PMID: 19542218 Free PMC article.
-
Aqueous access channels in subunit a of rotary ATP synthase.J Biol Chem. 2003 Feb 21;278(8):6066-74. doi: 10.1074/jbc.M210199200. Epub 2002 Dec 6. J Biol Chem. 2003. PMID: 12473663
-
Aqueous access pathways in ATP synthase subunit a. Reactivity of cysteine substituted into transmembrane helices 1, 3, and 5.J Biol Chem. 2007 Mar 23;282(12):9001-7. doi: 10.1074/jbc.M610848200. Epub 2007 Jan 18. J Biol Chem. 2007. PMID: 17234633
-
Structural model of the transmembrane Fo rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ.Biochim Biophys Acta. 2002 Oct 11;1565(2):232-45. doi: 10.1016/s0005-2736(02)00572-2. Biochim Biophys Acta. 2002. PMID: 12409198 Review.
-
Coupling proton movements to c-ring rotation in F(1)F(o) ATP synthase: aqueous access channels and helix rotations at the a-c interface.Biochim Biophys Acta. 2002 Sep 10;1555(1-3):29-36. doi: 10.1016/s0005-2728(02)00250-5. Biochim Biophys Acta. 2002. PMID: 12206887 Review.
Cited by
-
Constraining the Lateral Helix of Respiratory Complex I by Cross-linking Does Not Impair Enzyme Activity or Proton Translocation.J Biol Chem. 2015 Aug 21;290(34):20761-20773. doi: 10.1074/jbc.M115.660381. Epub 2015 Jul 1. J Biol Chem. 2015. PMID: 26134569 Free PMC article.
-
Structure of the c(10) ring of the yeast mitochondrial ATP synthase in the open conformation.Nat Struct Mol Biol. 2012 Apr 15;19(5):485-91, S1. doi: 10.1038/nsmb.2284. Nat Struct Mol Biol. 2012. PMID: 22504883 Free PMC article.
-
High-resolution structure of the rotor ring of a proton-dependent ATP synthase.Nat Struct Mol Biol. 2009 Oct;16(10):1068-73. doi: 10.1038/nsmb.1678. Epub 2009 Sep 27. Nat Struct Mol Biol. 2009. PMID: 19783985
-
Obstruction of transmembrane helical movements in subunit a blocks proton pumping by F1Fo ATP synthase.J Biol Chem. 2013 Aug 30;288(35):25535-25541. doi: 10.1074/jbc.M113.496794. Epub 2013 Jul 17. J Biol Chem. 2013. PMID: 23864659 Free PMC article.
-
Determinants of Directionality and Efficiency of the ATP Synthase Fo Motor at Atomic Resolution.J Phys Chem Lett. 2022 Jan 13;13(1):387-392. doi: 10.1021/acs.jpclett.1c03358. Epub 2022 Jan 5. J Phys Chem Lett. 2022. PMID: 34985899 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

