Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase
- PMID: 18332144
- PMCID: PMC2376249
- DOI: 10.1074/jbc.M709329200
Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase
Abstract
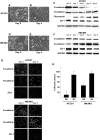
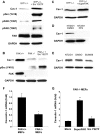
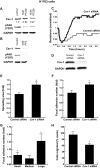
Emerging evidence has shown that caveolin-1 is up-regulated in a number of metastatic cancers and can influence various aspects of cell migration. However, in general, the role of caveolin-1 in cancer progression is poorly understood. In the present study, we examined alterations in caveolin-1 expression during epithelial-to-mesenchymal transition (EMT) and the ability of caveolin-1 to alter cancer cell adhesion, an aspect of cell motility. We employed two EMT cell models, the human embryonic carcinoma cell line NT2/D1, and TGF-beta1-treated NMuMG cells, which are derived from normal mouse mammary epithelia. Caveolin-1 expression was substantially up-regulated in both cell lines following the induction of EMT and was preceded by increased activation of focal adhesion kinase (FAK) and Src, two known tyrosine kinases involved in EMT. We hypothesized that caveolin-1 expression could be influenced by increased FAK phosphorylation, to which Src is a known contributor. Examination of FAK+/+ and FAK-/- mouse embryonic fibroblasts revealed that in cells devoid of FAK, caveolin-1 expression is strikingly diminished. Using FAK and superFAK constructs and the novel FAK inhibitor PF-228, we were able to demonstrate that indeed, FAK can regulate caveolin-1 expression. We also found that Src can contribute to increases in caveolin-1 expression, however, only in the presence of FAK. From the culmination of this data and our functional analyses, we conclude that caveolin-1 expression can be up-regulated during EMT, and further, once expressed, caveolin-1 can greatly influence cancer cell adhesion.
Figures



References
-
- Rothberg, K. G., Heuser, J. E., Donzell, W. C., Ying, Y. S., Glenney, J. R., and Anderson, R. G. (1992) Cell 68 673-682 - PubMed
-
- Glenney, J. R., Jr. (1989) J. Biol. Chem. 264 20163-20166 - PubMed
-
- Williams, T. M., and Lisanti, M. P. (2005) Am. J. Physiol. Cell Physiol. 288 C494-C506 - PubMed
-
- Hayashi, K., Matsuda, S., Machida, K., Yamamoto, T., Fukuda, Y., Nimura, Y., Hayakawa, T., and Hamaguchi, M. (2001) Cancer Res. 61 2361-2364 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

