Inhibitory effect of PRO 2000, a candidate microbicide, on dendritic cell-mediated human immunodeficiency virus transfer
- PMID: 18332174
- PMCID: PMC2346652
- DOI: 10.1128/AAC.00707-07
Inhibitory effect of PRO 2000, a candidate microbicide, on dendritic cell-mediated human immunodeficiency virus transfer
Abstract
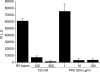
Without an effective vaccine against human immunodeficiency virus (HIV) infection, topical microbicide development has become a priority. The sulfonated polyanion PRO 2000, a candidate topical microbicide now in phase II/III clinical trials, blocks HIV infection of cervical tissue in vitro. Dendritic cells (DC) are among the first cell types to contact HIV in the genital tract and facilitate the spread of the virus. Thus, interfering with virus-DC interactions is a desirable characteristic of topical microbicides as long as that does not interfere with the normal function of DC. PRO 2000 present during capture of the replication-defective HIV(JRFL) reporter virus or replication-competent HIV(BaL) by monocyte-derived DC (MDDC) inhibited subsequent HIV transfer to target cells. Continuous exposure to PRO 2000 during MDDC-target cell coculture effectively inhibited HIV infection of target cells. PRO 2000 inhibited HIV capture by MDDC. In addition, the compound blocked R5 and X4 HIV envelope-mediated cell-cell fusion. Interestingly, simultaneous exposure to PRO 2000 and lipopolysaccharide attenuated the cytokine production in response to stimulation, suggesting that the compound altered DC function. While efficient blocking of MDDC-mediated virus transfer and infection in the highly permissive MDDC-T-cell environment reinforces the potential value of PRO 2000 as a topical microbicide against HIV, the impact of PRO 2000 on immune cell functions warrants careful evaluation.
Figures
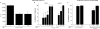


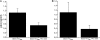

Similar articles
-
Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants.Retrovirology. 2006 Aug 1;3:46. doi: 10.1186/1742-4690-3-46. Retrovirology. 2006. PMID: 16882346 Free PMC article.
-
Attachment and fusion inhibitors potently prevent dendritic cell-driven HIV infection.J Acquir Immune Defic Syndr. 2011 Mar 1;56(3):204-12. doi: 10.1097/QAI.0b013e3181ff2aa5. J Acquir Immune Defic Syndr. 2011. PMID: 21084994 Free PMC article.
-
The candidate sulfonated microbicide, PRO 2000, has potential multiple mechanisms of action against HIV-1.Antiviral Res. 2009 Oct;84(1):38-47. doi: 10.1016/j.antiviral.2009.07.013. Epub 2009 Aug 5. Antiviral Res. 2009. PMID: 19664662
-
Clinical development of microbicides for the prevention of HIV infection.Curr Pharm Des. 2004;10(3):315-36. doi: 10.2174/1381612043386374. Curr Pharm Des. 2004. PMID: 14754390 Review.
-
Immunodeficiency virus exploitation of dendritic cells in the early steps of infection.J Leukoc Biol. 2003 Nov;74(5):683-90. doi: 10.1189/jlb.0403178. Epub 2003 Jul 22. J Leukoc Biol. 2003. PMID: 12960236 Review.
Cited by
-
In vitro evaluation of viability, integrity, and inflammation in genital epithelia upon exposure to pharmaceutical excipients and candidate microbicides.Antimicrob Agents Chemother. 2010 Dec;54(12):5105-14. doi: 10.1128/AAC.00456-10. Epub 2010 Oct 4. Antimicrob Agents Chemother. 2010. PMID: 20921308 Free PMC article.
-
HIV-1 Trans Infection of CD4(+) T Cells by Professional Antigen Presenting Cells.Scientifica (Cairo). 2013;2013:164203. doi: 10.1155/2013/164203. Epub 2013 May 7. Scientifica (Cairo). 2013. PMID: 24278768 Free PMC article. Review.
-
Targeting Trojan Horse leukocytes for HIV prevention.AIDS. 2010 Jan 16;24(2):163-87. doi: 10.1097/QAD.0b013e32833424c8. AIDS. 2010. PMID: 20010071 Free PMC article. Review. No abstract available.
-
A novel strategy for inducing enhanced mucosal HIV-1 antibody responses in an anti-inflammatory environment.PLoS One. 2011 Jan 6;6(1):e15861. doi: 10.1371/journal.pone.0015861. PLoS One. 2011. PMID: 21253014 Free PMC article.
-
Evaluation of WLBU2 peptide and 3-O-octyl-sn-glycerol lipid as active ingredients for a topical microbicide formulation targeting Chlamydia trachomatis.Antimicrob Agents Chemother. 2010 Feb;54(2):627-36. doi: 10.1128/AAC.00635-09. Epub 2009 Dec 14. Antimicrob Agents Chemother. 2010. PMID: 20008784 Free PMC article.
References
-
- Ambrose, Z., K. Larsen, J. Thompson, Y. Stevens, E. Finn, S. L. Hu, and M. L. Bosch. 2001. Evidence for early local viral replication and local production of antiviral immunity upon mucosal simian-human immunodeficiency virus SHIV89.6 infection in Macaca nemestrina. J. Virol. 75:8589-8596. - PMC - PubMed
-
- Anderson, R. A., K. A. Feathergill, X. H. Diao, M. D. Cooper, R. Kirkpatrick, B. C. Herold, G. F. Doncel, C. J. Chany, D. P. Waller, W. F. Rencher, and L. J. Zaneveld. 2002. Preclinical evaluation of sodium cellulose sulfate (Ushercell) as a contraceptive antimicrobial agent. J. Androl. 23:426-438. - PubMed
-
- Balzarini, J., Y. Van Herrewege, K. Vermeire, G. Vanham, and D. Schols. 2007. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol. Pharmacol. 71:3-11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

