A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology
- PMID: 18332178
- PMCID: PMC2275388
- DOI: 10.1084/jem.20071135
A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology
Abstract
Little is known about herpesvirus modulation of T cell activation in latently infected individuals or the implications of such for chronic immune disorders. Murine gammaherpesvirus 68 (MHV68) elicits persistent activation of CD8(+) T cells bearing a Vbeta4(+) T cell receptor (TCR) by a completely unknown mechanism. We show that a novel MHV68 protein encoded by the M1 gene is responsible for Vbeta4(+) CD8(+) T cell stimulation in a manner reminiscent of a viral superantigen. During infection, M1 expression induces a Vbeta4(+) effector T cell response that resists functional exhaustion and appears to suppress virus reactivation from peritoneal cells by means of long-term interferon-gamma (IFNgamma) production. Mice lacking an IFNgamma receptor (IFNgammaR(-/-)) fail to control MHV68 replication, and Vbeta4(+) and CD8(+) T cell activation by M1 instead contributes to severe inflammation and multiorgan fibrotic disease. Thus, M1 manipulates the host CD8(+) T cell response in a manner that facilitates latent infection in an immunocompetent setting, but promotes disease during a dysregulated immune response. Identification of a viral pathogenecity determinant with superantigen-like activity for CD8(+) T cells broadens the known repertoire of viral immunomodulatory molecules, and its function illustrates the delicate balance achieved between persistent viruses and the host immune response.
Figures




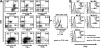


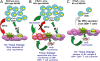
Similar articles
-
CD8+ T Cell Response to Gammaherpesvirus Infection Mediates Inflammation and Fibrosis in Interferon Gamma Receptor-Deficient Mice.PLoS One. 2015 Aug 28;10(8):e0135719. doi: 10.1371/journal.pone.0135719. eCollection 2015. PLoS One. 2015. PMID: 26317335 Free PMC article.
-
T cell reactivity during infectious mononucleosis and persistent gammaherpesvirus infection in mice.J Immunol. 2004 Mar 1;172(5):3078-85. doi: 10.4049/jimmunol.172.5.3078. J Immunol. 2004. PMID: 14978113
-
Effective control of chronic gamma-herpesvirus infection by unconventional MHC Class Ia-independent CD8 T cells.PLoS Pathog. 2006 May;2(5):e37. doi: 10.1371/journal.ppat.0020037. Epub 2006 May 19. PLoS Pathog. 2006. PMID: 16733540 Free PMC article.
-
Natural history of murine gamma-herpesvirus infection.Philos Trans R Soc Lond B Biol Sci. 2001 Apr 29;356(1408):569-79. doi: 10.1098/rstb.2000.0779. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11313012 Free PMC article. Review.
-
Insights into CD8 T Cell Activation and Exhaustion from a Mouse Gammaherpesvirus Model.Viral Immunol. 2020 Apr;33(3):215-224. doi: 10.1089/vim.2019.0183. Viral Immunol. 2020. PMID: 32286179 Free PMC article. Review.
Cited by
-
Viral-induced CD28 loss evokes costimulation independent alloimmunity.J Surg Res. 2015 Jun 15;196(2):241-6. doi: 10.1016/j.jss.2015.02.033. Epub 2015 Feb 21. J Surg Res. 2015. PMID: 25801976 Free PMC article.
-
Subcapsular sinus macrophages limit acute gammaherpesvirus dissemination.J Gen Virol. 2015 Aug;96(8):2314-2327. doi: 10.1099/vir.0.000140. Epub 2015 Apr 14. J Gen Virol. 2015. PMID: 25872742 Free PMC article.
-
Autoimmune melanocyte destruction is required for robust CD8+ memory T cell responses to mouse melanoma.J Clin Invest. 2011 May;121(5):1797-809. doi: 10.1172/JCI44849. Epub 2011 Apr 11. J Clin Invest. 2011. PMID: 21540555 Free PMC article.
-
BAFF receptor deficiency limits gammaherpesvirus infection.J Virol. 2014 Apr;88(8):3965-75. doi: 10.1128/JVI.03497-13. Epub 2014 Feb 5. J Virol. 2014. PMID: 24501409 Free PMC article.
-
Immune Control of γ-Herpesviruses.Viral Immunol. 2020 Apr;33(3):225-232. doi: 10.1089/vim.2019.0080. Epub 2019 Jul 22. Viral Immunol. 2020. PMID: 31334688 Free PMC article.
References
-
- Knipe, D.M., P.M. Howley, D.E. Griffin, R.A. Lamb, S.E. Straus, M.A. Martin, and B. Roizman, editors. 2007. Fields Virology. Lippincott Williams and Wilkins, Philadelphia.
-
- McGeoch, D.J., D. Gatherer, and A. Dolan. 2005. On phylogenetic relationships among major lineages of the Gammaherpesvirinae. J. Gen. Virol. 86:307–316. - PubMed
-
- Speck, S.H., and H.W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2:403–409. - PubMed
-
- Virgin, H.W., and S.H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11:371–379. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

