A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin
- PMID: 18332212
- PMCID: PMC2346692
- DOI: 10.1128/IAI.01424-07
A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin
Abstract
The antigenicity, structural location, and function of the predicted lipoprotein TP0136 of Treponema pallidum subsp. pallidum were investigated based on previous screening studies indicating that anti-TP0136 antibodies are present in the sera of syphilis patients and experimentally infected rabbits. Recombinant TP0136 (rTP0136) protein was purified and shown to be strongly antigenic during human and experimental rabbit infection. The TP0136 protein was exposed on the surface of the bacterial outer membrane and bound to the host extracellular matrix glycoproteins fibronectin and laminin. In addition, the TP0136 open reading frame was shown to be highly polymorphic among T. pallidum subspecies and strains at the nucleotide and amino acid levels. Finally, the ability of rTP0136 protein to act as a protective antigen to subsequent challenge with infectious T. pallidum in the rabbit model of infection was assessed. Immunization with rTP0136 delayed ulceration but did not prevent infection or the formation of lesions. These results demonstrate that TP0136 is expressed on the outer membrane of the treponeme during infection and may be involved in attachment to host extracellular matrix components.
Figures


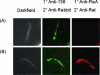
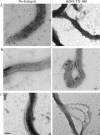

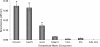
References
-
- Baker-Zander, S. A., E. W. Hook III, P. Bonin, H. H. Handsfield, and S. A. Lukehart. 1985. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J. Infect. Dis. 151264-272. - PubMed
-
- Blanco, D. R., C. I. Champion, A. Dooley, D. L. Cox, J. P. Whitelegge, K. Faull, and M. A. Lovett. 2005. A monoclonal antibody that conveys in vitro killing and partial protection in experimental syphilis binds a phosphorylcholine surface epitope of Treponema pallidum. Infect. Immun. 733083-3095. - PMC - PubMed
-
- Cameron, C. 2006. The T. pallidum outer membrane and outer membrane proteins, p. 237-266. In J. D. Radolf and S. Lukehart (ed.), Pathogenic Treponema. Caister Academic Press, Norfolk, VA.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

