Myoblasts and macrophages share molecular components that contribute to cell-cell fusion
- PMID: 18332221
- PMCID: PMC2265408
- DOI: 10.1083/jcb.200707191
Myoblasts and macrophages share molecular components that contribute to cell-cell fusion
Abstract
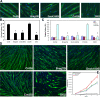
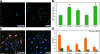
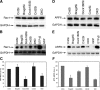
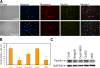
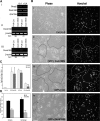
Cell-cell fusion is critical to the normal development of certain tissues, yet the nature and degree of conservation of the underlying molecular components remains largely unknown. Here we show that the two guanine-nucleotide exchange factors Brag2 and Dock180 have evolutionarily conserved functions in the fusion of mammalian myoblasts. Their effects on muscle cell formation are distinct and are a result of the activation of the GTPases ARF6 and Rac, respectively. Inhibition of ARF6 activity results in a lack of physical association between paxillin and beta(1)-integrin, and disruption of paxillin transport to sites of focal adhesion. We show that fusion machinery is conserved among distinct cell types because Dock180 deficiency prevented fusion of macrophages and the formation of multinucleated giant cells. Our results are the first to demonstrate a role for a single protein in the fusion of two different cell types, and provide novel mechanistic insight into the function of GEFs in the morphological maturation of multinucleated cells.
Figures



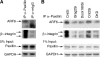

References
-
- Albert, M.L., J.I. Kim, and R.B. Birge. 2000. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2:899–905. - PubMed
-
- Brown, M.C., and C.E. Turner. 2004. Paxillin: adapting to change. Physiol. Rev. 84:1315–1339. - PubMed
-
- Charlton, C.A., W.A. Mohler, and H.M. Blau. 2000. Neural cell adhesion molecule (NCAM) and myoblast fusion. Dev. Biol. 221:112–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AG009521/AG/NIA NIH HHS/United States
- AG024987/AG/NIA NIH HHS/United States
- R01 EB005011/EB/NIBIB NIH HHS/United States
- AG020961/AG/NIA NIH HHS/United States
- AG009521/AG/NIA NIH HHS/United States
- T32 AI007328/AI/NIAID NIH HHS/United States
- AF051678/AF/ACF HHS/United States
- R01 AG020961/AG/NIA NIH HHS/United States
- R01 AG009521/AG/NIA NIH HHS/United States
- EB005011/EB/NIBIB NIH HHS/United States
- R01 HD018179/HD/NICHD NIH HHS/United States
- HD018179/HD/NICHD NIH HHS/United States
- T32 HD007249/HD/NICHD NIH HHS/United States
- R01 AG024987/AG/NIA NIH HHS/United States
- 5T32 HD007249/HD/NICHD NIH HHS/United States
- 5T32 AI07328/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

