Crystal structure of a full-length beta-catenin
- PMID: 18334222
- PMCID: PMC4267759
- DOI: 10.1016/j.str.2007.12.021
Crystal structure of a full-length beta-catenin
Abstract
beta-catenin plays essential roles in cell adhesion and Wnt signaling, while deregulation of beta-catenin is associated with multiple diseases including cancers. Here, we report the crystal structures of full-length zebrafish beta-catenin and a human beta-catenin fragment that contains both the armadillo repeat and the C-terminal domains. Our structures reveal that the N-terminal region of the C-terminal domain, a key component of the C-terminal transactivation domain, forms a long alpha helix that packs on the C-terminal end of the armadillo repeat domain, and thus forms part of the beta-catenin superhelical core. The existence of this helix redefines our view of interactions of beta-catenin with some of its critical partners, including ICAT and Chibby, which may form extensive interactions with this C-terminal domain alpha helix. Our crystallographic and NMR studies also suggest that the unstructured N-terminal and C-terminal tails interact with the ordered armadillo repeat domain in a dynamic and variable manner.
Figures

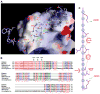


Comment in
-
Terminal regions of beta-catenin come into view.Structure. 2008 Mar;16(3):336-8. doi: 10.1016/j.str.2008.02.005. Structure. 2008. PMID: 18334207 Free PMC article.
References
-
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. - PubMed
-
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

