Sequence homology and microhomology dominate chromosomal double-strand break repair in African trypanosomes
- PMID: 18334531
- PMCID: PMC2377438
- DOI: 10.1093/nar/gkn104
Sequence homology and microhomology dominate chromosomal double-strand break repair in African trypanosomes
Abstract
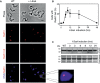
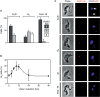
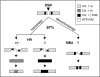
Genetic diversity in fungi and mammals is generated through mitotic double-strand break-repair (DSBR), typically involving homologous recombination (HR) or non-homologous end joining (NHEJ). Microhomology-mediated joining appears to serve a subsidiary function. The African trypanosome, a divergent protozoan parasite, relies upon rearrangement of subtelomeric variant surface glycoprotein (VSG) genes to achieve antigenic variation. Evidence suggests an absence of NHEJ but chromosomal repair remains largely unexplored. We used a system based on I-SceI meganuclease and monitored temporally constrained DSBR at a specific chromosomal site in bloodstream form Trypanosoma brucei. In response to the lesion, adjacent single-stranded DNA was generated; the homologous strand-exchange factor, Rad51, accumulated into foci; a G(2)M checkpoint was activated and >50% of cells displayed successful repair. Quantitative analysis of DSBR pathways employed indicated that inter-chromosomal HR dominated. HR displayed a strong preference for the allelic template but also the capacity to interact with homologous sequence on heterologous chromosomes. Intra-chromosomal joining was predominantly, and possibly exclusively, microhomology mediated, a situation unique among organisms examined to date. These DSBR pathways available to T. brucei likely underlie patterns of antigenic variation and the evolution of the vast VSG gene family.
Figures



References
-
- Simpson AG, Stevens JR, Lukes J. The evolution and diversity of kinetoplastid flagellates. Trends Parasitol. 2006;22:168–174. - PubMed
-
- Horn D. The molecular control of antigenic variation in Trypanosoma brucei. Curr. Mol. Med. 2004;4:563–576. - PubMed
-
- Morrison LJ, Majiwa P, Read AF, Barry JD. Probabilistic order in antigenic variation of Trypanosoma brucei. Int. J. Parasitol. 2005;35:961–972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

