The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline
- PMID: 18334580
- PMCID: PMC2386281
- DOI: 10.1210/jc.2008-0125
The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline
Abstract
Objective: The objective of the study was to develop clinical practice guidelines for the diagnosis of Cushing's syndrome.
Participants: The Task Force included a chair, selected by the Clinical Guidelines Subcommittee (CGS) of The Endocrine Society, five additional experts, a methodologist, and a medical writer. The Task Force received no corporate funding or remuneration.
Consensus process: Consensus was guided by systematic reviews of evidence and discussions. The guidelines were reviewed and approved sequentially by The Endocrine Society's CGS and Clinical Affairs Core Committee, members responding to a web posting, and The Endocrine Society Council. At each stage the Task Force incorporated needed changes in response to written comments.
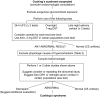
Conclusions: After excluding exogenous glucocorticoid use, we recommend testing for Cushing's syndrome in patients with multiple and progressive features compatible with the syndrome, particularly those with a high discriminatory value, and patients with adrenal incidentaloma. We recommend initial use of one test with high diagnostic accuracy (urine cortisol, late night salivary cortisol, 1 mg overnight or 2 mg 48-h dexamethasone suppression test). We recommend that patients with an abnormal result see an endocrinologist and undergo a second test, either one of the above or, in some cases, a serum midnight cortisol or dexamethasone-CRH test. Patients with concordant abnormal results should undergo testing for the cause of Cushing's syndrome. Patients with concordant normal results should not undergo further evaluation. We recommend additional testing in patients with discordant results, normal responses suspected of cyclic hypercortisolism, or initially normal responses who accumulate additional features over time.
Figures
References
-
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams Jr JW, Zaza S 2004 Grading quality of evidence and strength of recommendations. BMJ 328:1490 - PMC - PubMed
-
- Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, Ennis R, Erwin PJ, Montori VM 2008 Accuracy of diagnostic tests for Cushing syndrome: a systematic review and meta-analyses. J Clin Endocrinol Metab 93:1553–1562 - PubMed
-
- Swiglo BA, Murad MH, Schünemann HJ, Kunz R, Vigersky RA, Guyatt GH, Montori VM 2008 A case for clarity, consistency, and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the GRADE system. J Clin Endocrinol Metab 93:666–673 - PubMed
-
- Etxabe J, Vazquez JA 1994 Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf) 40:479–484 - PubMed
-
- Lindholm J, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jorgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J 2001 Incidence and late prognosis of Cushing's syndrome: a population-based study. J Clin Endocrinol Metab 86:117–123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

