Tel2 mediates activation and localization of ATM/Tel1 kinase to a double-strand break
- PMID: 18334620
- PMCID: PMC2279195
- DOI: 10.1101/gad.1646208
Tel2 mediates activation and localization of ATM/Tel1 kinase to a double-strand break
Abstract
The kinases ATM and ATR (Tel1 and Mec1 in the yeast Saccharomyces cerevisiae) control the response to DNA damage. We report that S. cerevisiae Tel2 acts at an early step of the TEL1/ATM pathway of DNA damage signaling. We show that Tel1 and Tel2 interact, and that even when Tel1 protein levels are high, this interaction is specifically required for Tel1 localization to a DNA break and its activation of downstream targets. Computational analysis revealed structural homology between Tel2 and Ddc2 (ATRIP in vertebrates), a partner of Mec1, suggesting a common structural principle used by partners of phoshoinositide 3-kinase-like kinases.
Figures
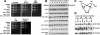


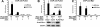
References
-
- Abraham R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Dev. 2001;15:2177–2196. - PubMed
-
- Ahmed S., Alpi A., Hengartner M.O., Gartner A. C. elegans RAD-5/CLK-2 defines a new DNA damage checkpoint protein. Curr. Biol. 2001;11:1934–1944. - PubMed
-
- Alcasabas A.A., Osborn A.J., Bachant J., Hu F., Werler P.J., Bousset K., Furuya K., Diffley J.F., Carr A.M., Elledge S.J. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 2001;3:958–965. - PubMed
-
- Brachmann C.B., Davies A., Cost G.J., Caputo E., Li J., Hieter P., Boeke J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. - PubMed
-
- Collis S.J., Barber L.J., Clark A.J., Martin J.S., Ward J.D., Boulton S.J. HCLK2 is essential for the mammalian S-phase checkpoint and impacts on Chk1 stability. Nat. Cell Biol. 2007;9:391–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
