General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis
- PMID: 18334669
- PMCID: PMC2329937
- DOI: 10.1105/tpc.107.054809
General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis
Abstract
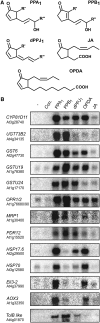
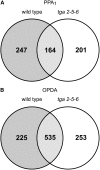
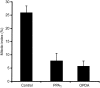
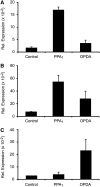
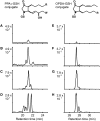
12-oxo-phytodienoic acid and several phytoprostanes are cyclopentenone oxylipins that are formed via the enzymatic jasmonate pathway and a nonenzymatic, free radical-catalyzed pathway, respectively. Both types of cyclopentenone oxylipins induce the expression of genes related to detoxification, stress responses, and secondary metabolism, a profile clearly distinct from that of the cyclopentanone jasmonic acid. Microarray analyses revealed that 60% of the induction by phytoprostanes and 30% of the induction by 12-oxo-phytodienoic acid was dependent on the TGA transcription factors TGA2, TGA5, and TGA6. Moreover, treatment with phytoprostanes and 12-oxo-phytodienoic acid inhibited cell division and root growth, a property also shared by jasmonic acid. Besides being potent signals, cyclopentenones and other lipid peroxidation products are reactive electrophiles that can covalently bind to and damage proteins. To this end, we show that at least two of the induced detoxification enzymes efficiently metabolize cyclopentenones in vitro. Accumulation of two of these metabolites was detectable during Pseudomonas infection. The cyclopentenone oxylipin gene induction profile resembles the defense response induced by a variety of lipophilic xenobiotics. Hence, oxidized lipids may activate chemosensory mechanisms of a general broad-spectrum detoxification network involving TGA transcription factors.
Figures

References
-
- Alary, J., Gueraud, F., and Cravedi, J.P. (2003). Fate of 4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol. Aspects Med. 24 177–187. - PubMed
-
- Almeras, E., Stolz, S., Vollenweider, S., Reymond, P., Mene-Saffrane, L., and Farmer, E.E. (2003). Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34 205–216. - PubMed
-
- An, Y.Q., McDowell, J.M., Huang, S., McKinney, E.C., Chambliss, S., and Meagher, R.B. (1996). Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10 107–121. - PubMed
-
- Baerson, S.R., Sanchez-Moreiras, A., Pedrol-Bonjoch, N., Schulz, M., Kagan, I.A., Agarwal, A.K., Reigosa, M.J., and Duke, S.O. (2005). Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J. Biol. Chem. 280 21867–21881. - PubMed
-
- Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 57 289–300.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

