Heparanase regulates retention and proliferation of primitive Sca-1+/c-Kit+/Lin- cells via modulation of the bone marrow microenvironment
- PMID: 18334674
- PMCID: PMC2964260
- DOI: 10.1182/blood-2007-10-116145
Heparanase regulates retention and proliferation of primitive Sca-1+/c-Kit+/Lin- cells via modulation of the bone marrow microenvironment
Abstract
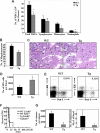
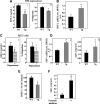
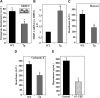
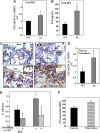
Heparanase is involved in tumor growth and metastasis. Because of its unique cleavage of heparan sulfate, which binds cytokines, chemokines and proteases, we hypothesized that heparanase is also involved in regulation of early stages of hematopoiesis. We report reduced numbers of maturing leukocytes but elevated levels of undifferentiated Sca-1(+)/c-Kit(+)/Lin(-) cells in the bone marrow (BM) of mice overexpressing heparanase (hpa-Tg). This resulted from increased proliferation and retention of the primitive cells in the BM microenvironment, manifested in increased SDF-1 turnover. Furthermore, heparanase overexpression in mice was accompanied by reduced protease activity of MMP-9, elastase, and cathepsin K, which regulate stem and progenitor cell mobilization. Moreover, increased retention of the progenitor cells also resulted from up-regulated levels of stem cell factor (SCF) in the BM, in particular in the stem cell-rich endosteum and endothelial regions. Increased SCF-induced adhesion of primitive Sca-1(+)/c-Kit(+)/Lin(-) cells to osteoblasts was also the result of elevation of the receptor c-Kit. Regulation of these phenomena is mediated by hyperphosphorylation of c-Myc in hematopoietic progenitors of hpa-Tg mice or after exogenous heparanase addition to wildtype BM cells in vitro. Altogether, our data suggest that heparanase modification of the BM microenvironment regulates the retention and proliferation of hematopoietic progenitor cells.
Figures
References
-
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. - PubMed
-
- Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. - PubMed
-
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. - PubMed
-
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. - PubMed
-
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

