Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet
- PMID: 18334714
- PMCID: PMC2740328
- DOI: 10.1126/stke.110pe13
Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet
Abstract
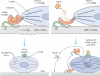
Soluble growth factors are potent regulators of normal and pathological processes. Mechanical factors are emerging as similarly important, but there has been no obvious mechanism linking the different factors. A recent report now demonstrates that cell-generated mechanical tension results in release of active transforming growth factor-beta from stiff extracellular matrix, providing a mechanism for differentiation and maintenance of myofibroblasts in processes like fibrosis. More broadly, the work suggests that matrix stiffness could regulate the equilibrium between storage and release of a host of matrix-bound growth factors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

