Mesenchymal cells from limbal stroma of human eye
- PMID: 18334960
- PMCID: PMC2268845
Mesenchymal cells from limbal stroma of human eye
Abstract
Purpose: Mesenchymal stem cells (MSC) are self-renewing, multipotent cells that are present in many adult tissues, including bone marrow, trabecular bone, adipose, and muscle. The presence of such cells of mesenchymal origin and their role during the wound healing of ocular injuries are currently being explored by many studies worldwide. In this study, we aimed to report the presence of mesenchymal cells resembling bone marrow-derived cells (MSC-BM) in the limbus of the human eye.
Methods: Fresh limbal tissues obtained from human subjects undergoing limbal biopsy for ocular surface reconstruction were used to establish limbal mesenchymal cell (MC-L) cultures. The spindle cell outgrowths observed in extended limbal epithelial cultures (LECs) and from deepithelialized limbal tissues were serially passaged using a human corneal epithelial (HCE) medium, which contained epidermal growth factor (EGF) and insulin, and supplemented with fetal bovine serum (FBS). MSC cultures were established from human bone marrow samples using Dulbecco's Modified Eagles Medium (DMEM) supplemented with FBS. The mesenchymal cells from both extended limbal cultures (MC-L) and bone marrow (MSC-BM) were characterized by morphology and immunophenotyping using epithelial, mesenchymal, hematopoietic, and endothelial markers using fluorescent-activated cell sorting (FACS). Selective markers were further confirmed by immunostaining and reverse transcription polymerase chain reaction (RT-PCR). Stromal cells of both origins (limbal and bone marrow-derived) were also evaluated for colony forming ability and population doubling. Attempts were made to differentiate these into adipocytes and osteocytes using conditioned medium.
Results: Spindle cells from extended limbal epithelial cultures as well as de-epithelialized human limbal tissues appeared elongated and fibroblast-like with oval vesicular nuclei. Both MC-L and MSC-BM showed colony forming ability in 14 days of plating. MC-L showed a population doubling of 22.95 while in MSC-BM, it was 30.98. Immunophenotyping of these cells by FACS and immunocytochemistry showed that the MC-L were positive for mesenchymal markers and negative for epithelial and hematopoietic markers similar to MSC-BM. The MC-L phenotype has thus been defined as MC-L(CD105, CD106, CD54, CD166, CD90, CD29, CD71, pax -6 +/ p75, SSEA1, Tra-1-61, Tra-1-81, CD31, CD34, CD45, CD11a, CD11c, CD14, CD138, Flk1, Flt1, VE-Cadherin -). The profile was further confirmed by RT-PCR. These cells also showed differentiation into adipocytes and osteocytes.
Conclusions: We demonstrated the presence of mesenchymal cells in the human limbus, similar to the bone marrow-derived MSC-BM. This presence suggests that these cells are unique to the adult stem cell niche.
Figures


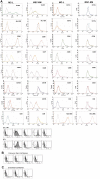

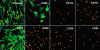



References
-
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–3. - PubMed
-
- Fatima A, Sangwan VS, Iftekhar G, Reddy P, Matalia H, Balasubramanian D, Vemuganti GK. Technique of cultivating limbal derived corneal epithelium on human amniotic membrane for clinical transplantation. J Postgrad Med. 2006;52:257–61. - PubMed
-
- Sangwan VS, Matalia HP, Vemuganti GK, Fatima A, Ifthekar G, Singh S, Nutheti R, Rao GN. Clinical outcome of autologous cultivated Limbal epithelium transplantation. Indian J Ophthalmol. 2006;54:29–34. - PubMed
-
- Tseng SC. Concept and application of Limbal stem cells. Eye. 1989;3:141–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
