Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding
- PMID: 18335973
- PMCID: PMC2740730
- DOI: 10.1021/cr050262p
Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding
Figures
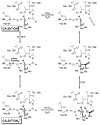



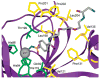
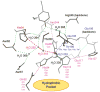





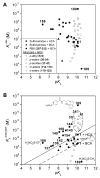
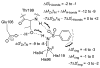
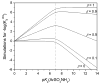


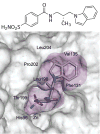
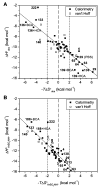


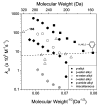
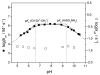


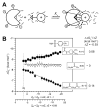
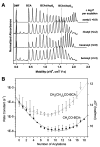

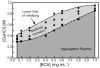

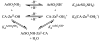
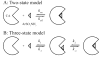
References
-
- Stams T, Christianson DW. In: The Carbonic Anhydrases: New Horizons. Chegwidden WR, Carter ND, Edwards YH, editors. Vol. 90 Birkhäuser Verlag: Basel, Switzerland; 2000.
-
- Supuran CT, Scozzafava A, Casini A. Med Res Rev. 2003;23:146. - PubMed
-
- Nishimori I, Minakuchi T, Kohsaki T, Onishi S, Takeuchi H, Vullo D, Scozzafava A, Supuran CT. Bioorg Med Chem Lett. 2007;17:3585. - PubMed
-
- Dill KA, Bromberg S. Molecular Driving Forces: Statistical Thermodynamics in Chemistry & Biology. Garland Science; New York: 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

