Mechanism and inhibition of saFabI, the enoyl reductase from Staphylococcus aureus
- PMID: 18335995
- PMCID: PMC4397500
- DOI: 10.1021/bi800023a
Mechanism and inhibition of saFabI, the enoyl reductase from Staphylococcus aureus
Abstract
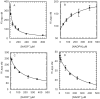
Approximately one-third of the world's population carries Staphylococcus aureus. The recent emergence of extreme drug resistant strains that are resistant to the "antibiotic of last resort", vancomycin, has caused a further increase in the pressing need to discover new drugs against this organism. The S. aureus enoyl reductase, saFabI, is a validated target for drug discovery. To drive the development of potent and selective saFabI inhibitors, we have studied the mechanism of the enzyme and analyzed the interaction of saFabI with triclosan and two related diphenyl ether inhibitors. Results from kinetic assays reveal that saFabI is NADPH-dependent, and prefers acyl carrier protein substrates carrying fatty acids with long acyl chains. On the basis of product inhibition studies, we propose that the reaction proceeds via an ordered sequential ternary complex, with the ACP substrate binding first, followed by NADPH. The interaction of NADPH with the enzyme has been further explored by site-directed mutagenesis, and residues R40 and K41 have been shown to be involved in determining the specificity of the enzyme for NADPH compared to NADH. Finally, in preliminary inhibition studies, we have shown that triclosan, 5-ethyl-2-phenoxyphenol (EPP), and 5-chloro-2-phenoxyphenol (CPP) are all nanomolar slow-onset inhibitors of saFabI. These compounds inhibit the growth of S. aureus with MIC values of 0.03-0.06 microg/mL. Upon selection for resistance, three novel safabI mutations, A95V, I193S, and F204S, were identified. Strains containing these mutations had MIC values approximately 100-fold larger than that of the wild-type strain, whereas the purified mutant enzymes had K i values 5-3000-fold larger than that of wild-type saFabI. The increase in both MIC and K i values caused by the mutations supports the proposal that saFabI is the intracellular target for the diphenyl ether-based inhibitors.
Figures



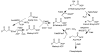

Similar articles
-
Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene.J Biol Chem. 2000 Feb 18;275(7):4654-9. doi: 10.1074/jbc.275.7.4654. J Biol Chem. 2000. PMID: 10671494
-
Rational optimization of drug-target residence time: insights from inhibitor binding to the Staphylococcus aureus FabI enzyme-product complex.Biochemistry. 2013 Jun 18;52(24):4217-28. doi: 10.1021/bi400413c. Epub 2013 Jun 6. Biochemistry. 2013. PMID: 23697754 Free PMC article.
-
Vibrio cholerae FabV defines a new class of enoyl-acyl carrier protein reductase.J Biol Chem. 2008 Jan 18;283(3):1308-1316. doi: 10.1074/jbc.M708171200. Epub 2007 Nov 21. J Biol Chem. 2008. PMID: 18032386
-
Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway.Acc Chem Res. 2008 Jan;41(1):11-20. doi: 10.1021/ar700156e. Acc Chem Res. 2008. PMID: 18193820 Review.
-
FabI (enoyl acyl carrier protein reductase) - A potential broad spectrum therapeutic target and its inhibitors.Eur J Med Chem. 2020 Dec 15;208:112757. doi: 10.1016/j.ejmech.2020.112757. Epub 2020 Aug 23. Eur J Med Chem. 2020. PMID: 32883635 Review.
Cited by
-
Staphylococcus epidermidis isolated in 1965 are more susceptible to triclosan than current isolates.PLoS One. 2013 Apr 16;8(4):e62197. doi: 10.1371/journal.pone.0062197. Print 2013. PLoS One. 2013. PMID: 23614034 Free PMC article.
-
A [(32)P]NAD(+)-based method to identify and quantitate long residence time enoyl-acyl carrier protein reductase inhibitors.Anal Biochem. 2015 Apr 1;474:40-9. doi: 10.1016/j.ab.2014.12.022. Epub 2015 Feb 14. Anal Biochem. 2015. PMID: 25684450 Free PMC article.
-
Discovery of a novel and potent class of F. tularensis enoyl-reductase (FabI) inhibitors by molecular shape and electrostatic matching.J Med Chem. 2012 Jan 12;55(1):268-79. doi: 10.1021/jm201168g. Epub 2011 Dec 5. J Med Chem. 2012. PMID: 22098466 Free PMC article.
-
Regulation of neuraminidase expression in Streptococcus pneumoniae.BMC Microbiol. 2012 Sep 11;12:200. doi: 10.1186/1471-2180-12-200. BMC Microbiol. 2012. PMID: 22963456 Free PMC article.
-
Treatment of Staphylococcus aureus with environmentally relevant concentrations of triclosan activates SaeRS-dependent virulence factor expression.Antimicrob Agents Chemother. 2025 Aug 6;69(8):e0172824. doi: 10.1128/aac.01728-24. Epub 2025 Jun 18. Antimicrob Agents Chemother. 2025. PMID: 40531055 Free PMC article.
References
-
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999, Clin. Infect. Dis. 2001;32(Suppl 2):S114–132. - PubMed
-
- Pfaller MA, Jones RN, Doern GV, Sader HS, Kugler KC, Beach ML. Survey of blood stream infections attributable to gram-positive cocci: frequency of occurrence and antimicrobial susceptibility of isolates collected in 1997 in the United States, Canada, and Latin America from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 1999;33:283–297. SENTRY Participants Group. - PubMed
-
- Peacock SJ, de Silva I, Lowy FD. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 2001;9:605–610. - PubMed
-
- Bradley SF. Methicillin-resistant Staphylococcus aureus infection. Clin. Geriatr. Med. 1992;8:853–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

