Creatine kinase-mediated ATP supply fuels actin-based events in phagocytosis
- PMID: 18336068
- PMCID: PMC2265766
- DOI: 10.1371/journal.pbio.0060051
Creatine kinase-mediated ATP supply fuels actin-based events in phagocytosis
Abstract
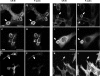
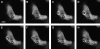
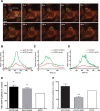
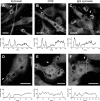
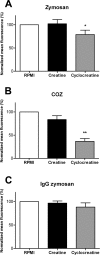
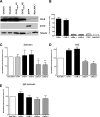
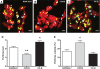
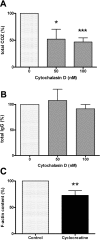
Phagocytosis requires locally coordinated cytoskeletal rearrangements driven by actin polymerization and myosin motor activity. How this actomyosin dynamics is dependent upon systems that provide access to ATP at phagosome microdomains has not been determined. We analyzed the role of brain-type creatine kinase (CK-B), an enzyme involved in high-energy phosphoryl transfer. We demonstrate that endogenous CK-B in macrophages is mobilized from the cytosolic pool and coaccumulates with F-actin at nascent phagosomes. Live cell imaging with XFP-tagged CK-B and beta-actin revealed the transient and specific nature of this partitioning process. Overexpression of a catalytic dead CK-B or CK-specific cyclocreatine inhibition caused a significant reduction of actin accumulation in the phagocytic cup area, and reduced complement receptor-mediated, but not Fc-gammaR-mediated, ingestion capacity of macrophages. Finally, we found that inhibition of CK-B affected phagocytosis already at the stage of particle adhesion, most likely via effects on actin polymerization behavior. We propose that CK-B activity in macrophages contributes to complement-induced F-actin assembly events in early phagocytosis by providing local ATP supply.
Conflict of interest statement
Figures
References
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
-
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
-
- Linehan SA, Martinez-Pomares L, Gordon S. Macrophage lectins in host defence. Microbes Infect. 2000;2:279–288. - PubMed
-
- Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76:231–248. - PubMed
-
- Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

