Lipoprotein size and susceptibility to atherosclerosis--insights from genetically modified mouse models
- PMID: 18336235
- PMCID: PMC3034977
- DOI: 10.2174/138945008783755629
Lipoprotein size and susceptibility to atherosclerosis--insights from genetically modified mouse models
Abstract
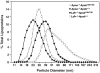
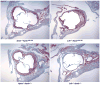
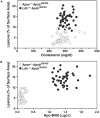
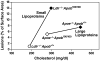
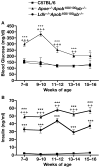
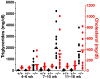
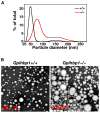
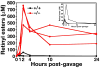
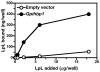
High plasma levels of the apo-B-containing lipoproteins are casually implicated in the pathogenesis of atherosclerosis. This finding, backed by decades of animal and human studies, has sparked interest in defining which classes of apo-B-containing lipoprotein particles are most atherogenic. Although small LDL particles and larger remnant lipoproteins both appear to be atherogenic, it has been difficult to discern which particles are the most atherogenic. Here, we summarize several mouse models that have provided insights into this issue. The influence of lipoprotein size on susceptibility to atherosclerosis was examined by studying the phenotypes of two strains of mice with virtually identical levels of plasma cholesterol--Ldlr(-/-)Apob(100/100) and Apoe(-/-) Apob(100/100) mice. The Ldlr(-/-) Apob(100/100) mice, where the cholesterol is in small LDL particles, had far more atherosclerosis than Apoe(-/-) Apob(100/100) mice, where virtually all of the cholesterol was in larger, VLDL-sized particles. Another intriguing animal model is the Gpihbp1-deficient mouse. GPIHBP1 is an endothelial cell platform for lipolysis, and mice lacking this protein have an accumulation of large, triglyceride-rich lipoproteins. Defining the extent of atherosclerosis in these mice should provide new insights into the atherogenicity of large, triglyceride-rich lipoproteins.
Figures





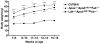






Similar articles
-
Lipoprotein size and atherosclerosis susceptibility in Apoe(-/-) and Ldlr(-/-) mice.Arterioscler Thromb Vasc Biol. 2001 Oct;21(10):1567-70. doi: 10.1161/hq1001.097780. Arterioscler Thromb Vasc Biol. 2001. PMID: 11597927 Review.
-
Defining the atherogenicity of large and small lipoproteins containing apolipoprotein B100.J Clin Invest. 2000 Dec;106(12):1501-10. doi: 10.1172/JCI10695. J Clin Invest. 2000. PMID: 11120757 Free PMC article.
-
LCAT modulates atherogenic plasma lipoproteins and the extent of atherosclerosis only in the presence of normal LDL receptors in transgenic rabbits.Arterioscler Thromb Vasc Biol. 2000 Feb;20(2):450-8. doi: 10.1161/01.atv.20.2.450. Arterioscler Thromb Vasc Biol. 2000. PMID: 10669643
-
Imbalance of APOB Lipoproteins and Large HDL in Type 1 Diabetes Drives Atherosclerosis.Circ Res. 2024 Jul 5;135(2):335-349. doi: 10.1161/CIRCRESAHA.123.323100. Epub 2024 Jun 3. Circ Res. 2024. PMID: 38828596 Free PMC article.
-
The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity.Curr Opin Lipidol. 2016 Oct;27(5):473-83. doi: 10.1097/MOL.0000000000000330. Curr Opin Lipidol. 2016. PMID: 27472409 Review.
Cited by
-
Novel Reversible Model of Atherosclerosis and Regression Using Oligonucleotide Regulation of the LDL Receptor.Circ Res. 2018 Feb 16;122(4):560-567. doi: 10.1161/CIRCRESAHA.117.311361. Epub 2018 Jan 10. Circ Res. 2018. PMID: 29321129 Free PMC article.
-
Predilection of Low Protein C-induced Spontaneous Atherothrombosis for the Right Coronary Sinus in Apolipoprotein E deficient mice.Sci Rep. 2018 Oct 10;8(1):15106. doi: 10.1038/s41598-018-32584-y. Sci Rep. 2018. PMID: 30305662 Free PMC article.
-
Distinct Influence of Hypercaloric Diets Predominant with Fat or Fat and Sucrose on Adipose Tissue and Liver Inflammation in Mice.Molecules. 2020 Sep 23;25(19):4369. doi: 10.3390/molecules25194369. Molecules. 2020. PMID: 32977558 Free PMC article.
-
Do the Apoe-/- and Ldlr-/- Mice Yield the Same Insight on Atherogenesis?Arterioscler Thromb Vasc Biol. 2016 Sep;36(9):1734-41. doi: 10.1161/ATVBAHA.116.306874. Epub 2016 Jul 7. Arterioscler Thromb Vasc Biol. 2016. PMID: 27386935 Free PMC article. Review.
-
Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins.Prog Retin Eye Res. 2009 Nov;28(6):393-422. doi: 10.1016/j.preteyeres.2009.08.001. Epub 2009 Aug 19. Prog Retin Eye Res. 2009. PMID: 19698799 Free PMC article. Review.
References
-
- Grundy SM. Cholesterol and coronary heart disease. A new era. J Am Med Assoc. 1986;256:2849–2858. - PubMed
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- NIH Consensus Development Panel. Triglyceride, high-density lipoprotein, and coronary heart disease. J Am Med Assoc. 1993;269:505–510. - PubMed
-
- Feussner G, Wagner A, Kohl B, Ziegler R. Clinical features of type III hyperlipoproteinemia: Analysis of 64 patients. Clin Investig. 1993;71:362–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
