Influence of supramammary lymph node extract on in vitro cell proliferation
- PMID: 18336474
- PMCID: PMC6496365
- DOI: 10.1111/j.1365-2184.2008.00521.x
Influence of supramammary lymph node extract on in vitro cell proliferation
Abstract
Objectives: Experiments were conducted to evaluate whether or not bovine supramammary lymph node extract (LNE) could support cell proliferation when it was substituted for bovine growth serum (BGS) in cell culture media.
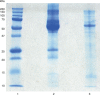
Materials and methods: Two different preparations of LNE were tested. The first yielded protein concentration of 3 mg/mL and the second contained 27 mg/mL protein. Three cell lines (MDA-MB-435, MAC-T and 1C6) were used in serum starvation assays to evaluate LNE. Cell proliferation assays were used to determine growth stimulation in the presence of LNE, and short-term or rapid adaptation cultures were evaluated for LNE effects on cell survival.
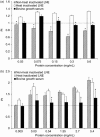
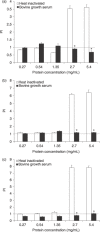
Results: Heat-inactivated preparation 1 supported cell proliferation as well as or better (12-39%) than BGS following 2 days of serum starvation in culture. The second lymph node preparation provided a stimulatory effect (263-702% greater than BGS across all cell lines) following serum starvation at 2.7 and 5.4 mg/mL protein supplementation. A gradual adaptation process with lymph node supplementation into media maintained cell population growth on a short-term basis. However, once cells were trypsinized or scraped and re-seeded into 2.7 mg/mL LNE protein containing media, cells were unable to re-adhere, leaving them detached, and eventually appearing to be dead.
Conclusions: Substitution of BGS with LNE protein dramatically stimulated cells to proliferate, but did not allow for rapid cell population growth adaptation in vitro.
Figures

References
-
- Akers RM (2006) Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows. J. Dairy Sci. 89, 1222–1234. - PubMed
-
- Berry SDK, Weber Nielsen MS, Sejresen K, Pearson RE, Boyle PL, Akers RM (2003) Use of immortalized bovine mammary epithelial cell line (MAC‐T) to measure the mitogenic activity of extracts from heifer mammary tissue: effects of nutrition and ovariectomy. Domest. Anim. Endocrinol. 25, 245–253. - PubMed
-
- Bradley KJ, Bradley AJ, Barr FJ (2001) Ultrasonographic appearance of the superficial supramammary lymph nodes in lactating dairy cattle. Vet. Rec. 148, 497–501. - PubMed
-
- Carroll MC (2004) The complement system in regulation of adaptive immunity. Nat. Immunol. 5, 981–986. - PubMed
-
- Gorewit RC, Ostensson K, Astrom G, Svennerstenz. K (1993) Flow and composition of afferent mammary gland lymph. J. Dairy Sci. 76, 1539–1543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

