Tissue-targeted therapy of autoimmune diabetes using dendritic cells transduced to express IL-4 in NOD mice
- PMID: 18337172
- PMCID: PMC2453076
- DOI: 10.1016/j.clim.2007.12.009
Tissue-targeted therapy of autoimmune diabetes using dendritic cells transduced to express IL-4 in NOD mice
Abstract
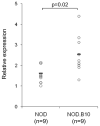
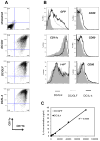
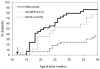
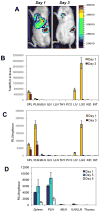
A deficit in IL-4 production has been previously reported in both diabetic human patients and non-obese diabetic (NOD) mice. In addition, re-introducing IL-4 into NOD mice systemically, or as a transgene, led to a beneficial outcome in most studies. Here, we show that prediabetic, 12-week old female NOD mice have a deficit in IL-4 expression in the pancreatic lymph nodes (PLN) compared to age-matched diabetes-resistant NOD.B10 mice. By bioluminescence imaging, we demonstrated that the PLN was preferentially targeted by bone marrow-derived dendritic cells (DCs) following intravenous (IV) administration. Following IV injection of DCs transduced to express IL-4 (DC/IL-4) into 12-week old NOD mice, it was possible to significantly delay or prevent the onset of hyperglycemia. We then focused on the PLN to monitor, by microarray analysis, changes in gene expression induced by DC/IL-4 and observed a rapid normalization of the expression of many genes, that were otherwise under-expressed compared to NOD.B10 PLN. The protective effect of DC/IL-4 required both MHC and IL-4 expression by the DCs. Thus, adoptive cellular therapy, using DCs modified to express IL-4, offers an effective, tissue-targeted cellular therapy to prevent diabetes in NOD mice at an advanced stage of pre-diabetes, and may offer a safe approach to consider for treatment of high risk human pre-diabetic patients.
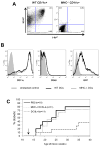
Figures

References
-
- Shoda L, Young D, Ramanujan S, Whiting C, Atkinson M, Bluestone J, Eisenbarth G, Mathis D, Rossini A, Campbell S, Kahn R, Kreuwel H. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–26. - PubMed
-
- Roep B, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol. 2004;4:989–97. - PubMed
-
- Creusot R, Fathman CG, Muller-Ladner U, Tarner I. Targeted gene therapy of autoimmune diseases: advances and prospects. Expert Rev Clin Immunol. 2005;1:385–404. - PubMed
-
- Cameron M, Arreaza G, Zucker P, Chensue S, Strieter R, Chakrabarti S, Delovitch T. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159:4686–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

