Novel role for STIM1 as a trigger for calcium influx factor production
- PMID: 18337241
- PMCID: PMC2386937
- DOI: 10.1074/jbc.M709575200
Novel role for STIM1 as a trigger for calcium influx factor production
Abstract
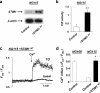
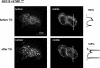
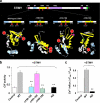
STIM1 has been recently identified as a Ca(2+) sensor in endoplasmic reticulum (ER) and an initiator of the store-operated Ca(2+) entry (SOCE) pathway, but the mechanism of SOCE activation remains controversial. Here we focus on the early ER-delimited steps of the SOCE pathway and demonstrate that STIM1 is critically involved in initiating of production of calcium influx factor (CIF), a diffusible messenger that can deliver the signal from the stores to plasma membrane and activate SOCE. We discovered that CIF production is tightly coupled with STIM1 expression and requires functional integrity of its intraluminal sterile alpha-motif (SAM) domain. We demonstrate that 1) molecular knockdown or overexpression of STIM1 results in corresponding impairment or amplification of CIF production and 2) inherent deficiency in the ER-delimited CIF production and SOCE activation in some cell types can be a result of their deficiency in STIM1 protein; expression of a wild-type STIM1 in such cells was sufficient to fully rescue their ability to produce CIF and SOCE. We found that glycosylation sites in the ER-resident SAM domain of STIM1 are essential for initiation of CIF production. We propose that after STIM1 loses Ca(2+) from EF hand, its intraluminal SAM domain may change conformation, and via glycosylation sites it can interact with and activate CIF-producing machinery. Thus, CIF production appears to be one of the earliest STIM1-dependent events in the ER lumen, and impairment of this process results in impaired SOCE response.
Figures
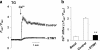



Similar articles
-
The polybasic lysine-rich domain of plasma membrane-resident STIM1 is essential for the modulation of store-operated divalent cation entry by extracellular calcium.Cell Signal. 2013 May;25(5):1328-37. doi: 10.1016/j.cellsig.2013.01.025. Epub 2013 Feb 8. Cell Signal. 2013. PMID: 23395841
-
Intramolecular shielding maintains the ER Ca²⁺ sensor STIM1 in an inactive conformation.J Cell Sci. 2013 Jun 1;126(Pt 11):2401-10. doi: 10.1242/jcs.117200. Epub 2013 Apr 9. J Cell Sci. 2013. PMID: 23572507
-
Structural perturbations induced by Asn131 and Asn171 glycosylation converge within the EFSAM core and enhance stromal interaction molecule-1 mediated store operated calcium entry.Biochim Biophys Acta Mol Cell Res. 2017 Jun;1864(6):1054-1063. doi: 10.1016/j.bbamcr.2016.11.015. Epub 2016 Nov 17. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27865927
-
Ca2+ signaling and STIM1.Prog Biophys Mol Biol. 2010 Sep;103(1):51-8. doi: 10.1016/j.pbiomolbio.2010.02.004. Epub 2010 Mar 11. Prog Biophys Mol Biol. 2010. PMID: 20226808 Review.
-
Remodelling of the endoplasmic reticulum during store-operated calcium entry.Biol Cell. 2011 Aug;103(8):365-80. doi: 10.1042/BC20100152. Biol Cell. 2011. PMID: 21736554 Review.
Cited by
-
Plasma Membrane and Organellar Targets of STIM1 for Intracellular Calcium Handling in Health and Neurodegenerative Diseases.Cells. 2021 Sep 23;10(10):2518. doi: 10.3390/cells10102518. Cells. 2021. PMID: 34685498 Free PMC article. Review.
-
Orai1 and Ca2+-independent phospholipase A2 are required for store-operated Icat-SOC current, Ca2+ entry, and proliferation of primary vascular smooth muscle cells.Am J Physiol Cell Physiol. 2012 Mar 1;302(5):C748-56. doi: 10.1152/ajpcell.00312.2011. Epub 2011 Nov 16. Am J Physiol Cell Physiol. 2012. PMID: 22094335 Free PMC article.
-
SOC: now also store-operated cyclase.Nat Cell Biol. 2009 Apr;11(4):381-2. doi: 10.1038/ncb0409-381. Nat Cell Biol. 2009. PMID: 19337322
-
Mutations of the Ca2+-sensing stromal interaction molecule STIM1 regulate Ca2+ influx by altered oligomerization of STIM1 and by destabilization of the Ca2+ channel Orai1.J Biol Chem. 2013 Jan 18;288(3):1653-64. doi: 10.1074/jbc.M112.417246. Epub 2012 Dec 4. J Biol Chem. 2013. PMID: 23212906 Free PMC article.
-
STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice.J Clin Invest. 2012 Jun;122(6):2267-82. doi: 10.1172/JCI59953. Epub 2012 May 1. J Clin Invest. 2012. PMID: 22546859 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

