The cytoplasmic loops of subunit a of Escherichia coli ATP synthase may participate in the proton translocating mechanism
- PMID: 18337242
- PMCID: PMC2442323
- DOI: 10.1074/jbc.M800900200
The cytoplasmic loops of subunit a of Escherichia coli ATP synthase may participate in the proton translocating mechanism
Abstract
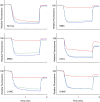
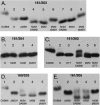
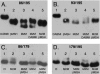
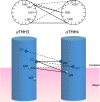
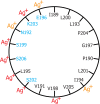
Subunit a plays a key role in promoting H(+) transport and the coupled rotary motion of the subunit c ring in F(1)F(0)-ATP synthase. H(+) binding and release occur at Asp-61 in the middle of the second transmembrane helix (TMH) of F(0) subunit c. H(+) are thought to reach Asp-61 via aqueous pathways mapping to the surfaces of TMHs 2-5 of subunit a based upon the chemical reactivity of Cys substituted into these helices. Here we substituted Cys into loops connecting TMHs 1 and 2 (loop 1-2) and TMHs 3 and 4 (loop 3-4). A large segment of loop 3-4 extending from loop residue 192 loop to residue 203 in TMH4 at the lipid bilayer surface proved to be very sensitive to inhibition by Ag(+). Cys-161 and -165 at the other end of the loop bordering TMH3 were also sensitive to inhibition by Ag(+). Further Cys substitutions in residues 86 and 93 in the middle of the 1-2 loop proved to be Ag(+)-sensitive. We next asked whether the regions of Ag(+)-sensitive residues clustered together near the surface of the membrane by combining Cys substitutions from two domains and testing for cross-linking. Cys-161 and -165 in loop 3-4 were found to cross-link with Cys-202, -203, or -205, which extend into TMH4 from the cytoplasm. Further Cys at residues 86 and 93 in loop 1-2 were found to cross-link with Cys-195 in loop 3-4. We conclude that the Ag(+)-sensitive regions of loops 1-2 and 3-4 may pack in a single domain that packs at the ends of TMHs 3 and 4. We suggest that the Ag(+)-sensitive domain may be involved in gating H(+) release at the cytoplasmic side of the aqueous access channel extending through F(0).
Figures


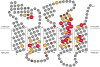
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

