Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity
- PMID: 18337273
- PMCID: PMC2657201
- DOI: 10.1093/brain/awn025
Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity
Abstract
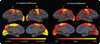
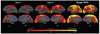
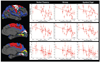
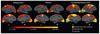
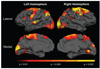
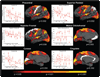
The clinical phenotype of Huntington's disease (HD) is far more complex and variable than depictions of it as a progressive movement disorder dominated by neostriatal pathology represent. The availability of novel neuro-imaging methods has enabled us to evaluate cerebral cortical changes in HD, which we have found to occur early and to be topographically selective. What is less clear, however, is how these changes influence the clinical expression of the disease. In this study, we used a high-resolution surface based analysis of in vivo MRI data to measure cortical thickness in 33 individuals with HD, spanning the spectrum of disease and 22 age- and sex-matched controls. We found close relationships between specific functional and cognitive measures and topologically specific cortical regions. We also found that distinct motor phenotypes were associated with discrete patterns of cortical thinning. The selective topographical associations of cortical thinning with clinical features of HD suggest that we are not simply correlating global worsening with global cortical degeneration. Our results indicate that cortical involvement contributes to important symptoms, including those that have been ascribed primarily to the striatum, and that topologically selective changes in the cortex might explain much of the clinical heterogeneity found in HD. Additionally, a significant association between regional cortical thinning and total functional capacity, currently the leading primary outcome measure used in neuroprotection trials for HD, establishes cortical MRI morphometry as a potential biomarker of disease progression.
Figures


References
-
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. - PubMed
-
- Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ. Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology. 1994;44:823–828. - PubMed
-
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, et al. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci. 2000;12:988–1000. - PubMed
-
- Billingsley RL, Simos PG, Castillo EM, Sarkari S, Breier JI, Pataraia E, et al. Spatio-temporal cortical dynamics of phonemic and semantic fluency. J Clin Exp Neuropsychol. 2004;26:1031–1043. - PubMed
-
- Boecker H, Ceballos-Baumann A, Bartenstein P, Weindl A, Siebner HR, Fassbender T, et al. Sensory processing in Parkinson’s and Huntington’s disease: investigations with 3D H(2)(15)O- PET. Brain. 1999;122(Pt 9):1651–1665. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

