Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma
- PMID: 18337403
- PMCID: PMC6670674
- DOI: 10.1523/JNEUROSCI.4443-07.2008
Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma
Abstract
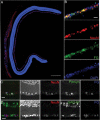
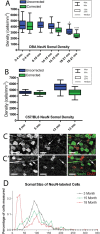
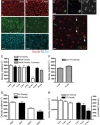
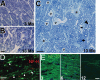
Glaucoma is characterized by retinal ganglion cell (RGC) pathology and a progressive loss of vision. Previous studies suggest RGC death is responsible for vision loss in glaucoma, yet evidence from other neurodegenerative diseases suggests axonal degeneration, in the absence of neuronal loss, can significantly affect neuronal function. To characterize RGC degeneration in the DBA/2 mouse model of glaucoma, we quantified RGCs in mice of various ages using neuronal-specific nuclear protein (NeuN) immunolabeling, retrograde labeling, and optic nerve axon counts. Surprisingly, the number of NeuN-labeled RGCs did not decline significantly until 18 months of age, at which time a significant decrease in RGC somal size was also observed. Axon dysfunction and degeneration occurred before loss of NeuN-positive RGCs, because significant declines in RGC number assayed by retrograde tracers and axon counts were observed at 13 months. To examine whether axonal dysfunction/degeneration affected gene expression in RGC axons or somas, NeuN and neurofilament-heavy (NF-H) immunolabeling was performed along with quantitative reverse transcription-PCR for RGC-specific genes in retinas of aged DBA/2 mice. Although these mice had similar numbers of NeuN-positive RGCs, the expression of neurofilament light, Brn-3b, and Sncg mRNA varied; this variation in RGC-specific gene expression was correlated with the appearance of NF-H immunoreactive RGC axons. Together, these data support a progression of RGC degeneration in this model of glaucoma, beginning with loss of retrograde label, where axon dysfunction and degeneration precede neuronal loss. This progression of degeneration suggests a need to examine the RGC axon as a locus of pathology in glaucoma.
Figures

References
-
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. - PubMed
-
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–409. - PubMed
-
- Chen YG, Zhang C, Chiang SK, Wu T, Tso MO. Increased nuclear factor-kappa B p65 immunoreactivity following retinal ischemia and reperfusion injury in mice. J Neurosci Res. 2003;72:125–131. - PubMed
-
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
