Adaptive control of saccades via internal feedback
- PMID: 18337410
- PMCID: PMC2733833
- DOI: 10.1523/JNEUROSCI.5300-07.2008
Adaptive control of saccades via internal feedback
Abstract
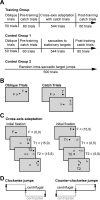
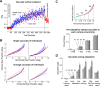
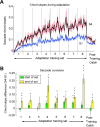
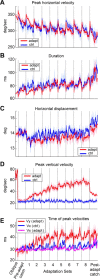
Ballistic movements like saccades require the brain to generate motor commands without the benefit of sensory feedback. Despite this, saccades are remarkably accurate. Theory suggests that this accuracy arises because the brain relies on an internal forward model that monitors the motor commands, predicts their sensory consequences, and corrects eye trajectory midflight. If control of saccades relies on a forward model, then the forward model should adapt whenever its predictions fail to match sensory feedback at the end of the movement. Using optimal feedback control theory, we predicted how this adaptation should alter saccade trajectories. We trained subjects on a paradigm in which the horizontal target jumped vertically during the saccade. With training, the final position of the saccade moved toward the second target. However, saccades became increasingly curved, i.e., suboptimal, as oculomotor commands were corrected on-line to steer the eye toward the second target. The adaptive response had two components: (1) the motor commands that initiated the saccades changed slowly, aiming the saccade closer to the jumped target. The adaptation of these earliest motor commands displayed little forgetting during the rest periods. (2) Late in saccade trajectory, another adaptive response steered it still closer to the jumped target, producing curvature. Adaptation of these late motor commands showed near-complete forgetting during the rest periods. The two components adapted at different timescales, with the late-acting component displaying much faster rates. It appears that in controlling saccades, the brain relies on an internal feedback that has the characteristics of a fast-adapting forward model.
Figures

Comment in
-
Optimally straight and optimally curved saccades.J Neurosci. 2008 Jul 23;28(30):7455-7. doi: 10.1523/JNEUROSCI.1817-08.2008. J Neurosci. 2008. PMID: 18650323 Free PMC article. No abstract available.
References
-
- Boehnke SE, Berg DJ, Marino RA, Itti L, Munoz DP. Adaptation, habituation and dishabituation of visual responses in the superior colliculus. Soc Neurosci Abstr. 2007;33:617–12.
-
- Catz N, Dicke PW, Thier P. The compensation of saccadic fatigue is based on the adjustment of a Purkinje cell simple spike population. Soc Neurosci Abstr. 2006;32:345.22/S17.
-
- Deubel H. Adaptivity of gain and direction in oblique saccades. In: O'Regan JK, Levy-Schoen A, editors. Eye movements: from physiology to cognition. New York: Elsevier; 1987. pp. 181–190.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
