The mitochondrial protease AFG3L2 is essential for axonal development
- PMID: 18337413
- PMCID: PMC6670688
- DOI: 10.1523/JNEUROSCI.4677-07.2008
The mitochondrial protease AFG3L2 is essential for axonal development
Abstract
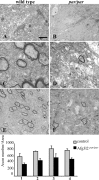
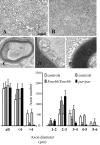
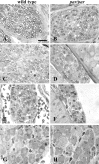
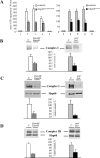
The mitochondrial metalloprotease AFG3L2 assembles with the homologous protein paraplegin to form a supracomplex in charge of the essential protein quality control within mitochondria. Mutations of paraplegin cause a specific axonal degeneration of the upper motoneuron and, therefore, hereditary spastic paraplegia. Here we present two Afg3l2 murine models: a newly developed null and a spontaneous mutant that we found carrier of a missense mutation. Contrasting with the mild and late onset axonal degeneration of paraplegin-deficient mouse, Afg3l2 models display a marked impairment of axonal development with delayed myelination and poor axonal radial growth leading to lethality at P16. The increased severity of the Afg3l2 mutants is explained by two main molecular features that differentiate AFG3L2 from paraplegin: its higher neuronal expression and its versatile ability to support both hetero-oligomerization and homo-oligomerization. Our data assign to AFG3L2 a crucial role by linking mitochondrial metabolism and axonal development. Moreover, we propose AFG3L2 as an excellent candidate for motoneuron and cerebellar diseases with early onset unknown etiology.
Figures




References
-
- Blondet B, Duxson MJ, Harris AJ, Melki J, Guenet JL, Pincon-Raymond M, Rieger F. Nerve and muscle development in paralyse mutant mice. Dev Biol. 1989;132:153–166. - PubMed
-
- Bross P, Rugarli EI, Casari G, Langer T. Protein quality control in mitochondria and neurodegeneration in hereditary spastic paraplegia. Heidelberg: Springer; 2004.
-
- Casari G, De Fusco M, Ciarmatori S, Zeviani M, Mora M, Fernandez P, De Michele G, Filla A, Cocozza S, Marconi R, Dürr A, Fontaine B, Ballabio A. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell. 1998;93:973–983. - PubMed
-
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
