Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles
- PMID: 18337417
- PMCID: PMC6670663
- DOI: 10.1523/JNEUROSCI.4063-07.2008
Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles
Abstract
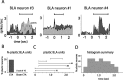
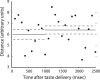
Emotional learning requires the coordinated action of neural populations in limbic and cortical networks. Here, we performed simultaneous extracellular recordings from gustatory cortical (GC) and basolateral amygdalar (BLA) neural ensembles as awake, behaving rats learned to dislike the taste of saccharin [via conditioned taste aversion (CTA)]. Learning-related changes in single-neuron sensory responses were observed in both regions, but the nature of the changes was region specific. In GC, most changes were restricted to relatively late aspects of the response (starting approximately 1.0 s after stimulus administration), supporting our hypothesis that in this paradigm palatability-related information resides exclusively in later cortical responses. In contrast, and consistent with data suggesting the amygdala's primary role in judging stimulus palatability, CTA altered all components of BLA taste responses, including the earliest. Finally, learning caused dramatic increases in the functional connectivity (measured in terms of cross-correlation peak heights) between pairs of simultaneously recorded BLA and GC neurons, increases that were evident only during taste processing. Our simultaneous assays of the activity of single neurons in multiple relevant brain regions across learning suggest that the transmission of taste information through amygdala-cortical circuits plays a vital role in CTA memory formation.
Figures








References
-
- Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. - PubMed
-
- Bermudez-Rattoni F, Grijalva CV, Kiefer SW, Garcia J. Flavor-illness aversions: the role of the amygdala in the acquisition of taste-potentiated odor aversions. Physiol Behav. 1986;38:503–508. - PubMed
-
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
