Long-term efficacy and safety of a calcineurin inhibitor-free regimen in live-donor renal transplant recipients
- PMID: 18337483
- PMCID: PMC2396928
- DOI: 10.1681/ASN.2007091001
Long-term efficacy and safety of a calcineurin inhibitor-free regimen in live-donor renal transplant recipients
Abstract
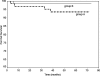
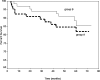
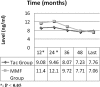
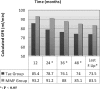
Calcineurin inhibitor (CNI) nephrotoxicity is a major concern after renal transplantation. To investigate the safety and efficacy of a CNI-free immunosuppressive regimen, 132 live-donor renal transplant recipients were included in a prospective, randomized controlled trial. All patients received induction therapy with basiliximab and steroids. The patients were randomized to a maintenance immunosuppression regimen that included steroids, sirolimus, and either low-dose tacrolimus or mycophenolate mofetil (MMF). Over a mean follow-up period of approximately 5 yr, patient and graft survival did not significantly differ between the two maintenance regimens. Patient survival was 93.8% and 98.5% in the tacrolimus/sirolimus and MMF/sirolimus groups, respectively, and graft survival was 83% and 88%, respectively. However, the MMF/sirolimus group had significantly better renal function, calculated by Cockcroft-Gault, from the second year post-transplant until the last follow-up. In addition, this group was less likely to require a change in their primary immunosuppression regimen than the tacrolimus/sirolimus group (20.8% versus 53.8%, P = 0.001). The safety profile was similar between groups. In summary, after long-term follow-up, a CNI-free maintenance regimen consisting of sirolimus, MMF, and steroids was both safe and efficacious among low to moderate immunologic risk renal transplant recipients.
Figures

References
-
- Myers BD: Cyclosporine nephrotoxicity. Kidney Int 30: 964–974, 1986 - PubMed
-
- Remuzzi G, Bertani T: Renal vascular and thrombotic effects of cyclosporine. Am J Kidney Dis 13: 261–272, 1989 - PubMed
-
- Bennett WM, Houghton DC, Buss WC: Cyclosporine-induced renal dysfunction: Correlations between cellular events and whole kidney function. J Am Soc Nephrol 1: 1212–1219, 1991 - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, O'squosquo; apos; yConnell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 - PubMed
-
- Ducloux D, Fournier V, Bresson-Vautrin C, Rebibou JM, Billerey C, Saint-Hiller Y, Chalpoin JM: Mycophenolate mofetil in renal transplant recipients with cyclosporine-associated nephrotoxicity: A preliminary report. Transplantation 65: 1504–1506, 1998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

