Role of integrins in the assembly and function of hensin in intercalated cells
- PMID: 18337486
- PMCID: PMC2396932
- DOI: 10.1681/ASN.2007070737
Role of integrins in the assembly and function of hensin in intercalated cells
Abstract
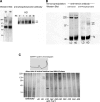
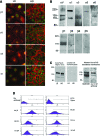
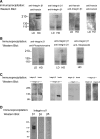
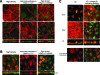
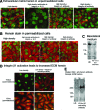
Epithelial differentiation proceeds in at least two steps: Conversion of a nonepithelial cell into an epithelial sheet followed by terminal differentiation into the mature epithelial phenotype. It was recently discovered that the extracellular matrix (ECM) protein hensin is able to convert a renal intercalated cell line from a flat, squamous shape into a cuboidal or columnar epithelium. Global knockout of hensin in mice results in embryonic lethality at the time that the first columnar cells appear. Here, antibodies that either activate or block integrin beta1 were used to demonstrate that activation of integrin alpha v beta 1 causes deposition of hensin in the ECM. Once hensin polymerizes and deposits into the ECM, it binds to integrin alpha 6 and mediates the conversion of epithelial cells to a cuboidal phenotype capable of apical endocytosis; therefore, multiple integrins play a role in the terminal differentiation of the intercalated cell: alpha v beta 1 generates polymerized hensin, and another set of integrins (containing alpha 6) mediates signals between hensin and the interior of the cells.
Figures

Comment in
-
Integrins, extracellular matrix, and terminal differentiation of renal epithelial cells.J Am Soc Nephrol. 2008 Jun;19(6):1043-4. doi: 10.1681/ASN.2008040370. Epub 2008 May 14. J Am Soc Nephrol. 2008. PMID: 18480310 No abstract available.
References
-
- Vijayakumar S, Takito J, Gao X, Schwartz GJ, Al-Awqati Q: Differentiation of columnar epithelia: The hensin pathway. J Cell Science 119: 4797–4801, 2006 - PubMed
-
- Schwartz GJ, Barasch J, Al-Awqati Q: Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 - PubMed
-
- Edwards JC, van Adelsberg J, Rater M, Herzlinger D, Lebowitz J, Al-Awqati Q: Conditional immortalization of bicarbonate-secreting intercalated cells from rabbit. Am J Physiol 263: C521–C529, 1992 - PubMed
-
- van Adelsberg J, Edwards JC, Takito J, Kiss B, Al-Awqati Q: An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell 76: 1053–1061, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

