Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms
- PMID: 18337499
- PMCID: PMC2393760
- DOI: 10.1073/pnas.0707370105
Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms
Abstract
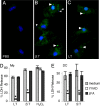
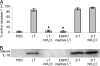
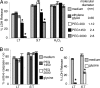
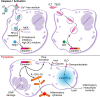
Caspase-1 cleaves the inactive IL-1beta and IL-18 precursors into active inflammatory cytokines. In Salmonella-infected macrophages, caspase-1 also mediates a pathway of proinflammatory programmed cell death termed "pyroptosis." We demonstrate active caspase-1 diffusely distributed in the cytoplasm and localized in discrete foci within macrophages responding to either Salmonella infection or intoxication by Bacillus anthracis lethal toxin (LT). Both stimuli triggered caspase-1-dependent lysis in macrophages and dendritic cells. Activation of caspase-1 by LT required binding, uptake, and endosome acidification to mediate translocation of lethal factor (LF) into the host cell cytosol. Catalytically active LF cleaved cytosolic substrates and activated caspase-1 by a mechanism involving proteasome activity and potassium efflux. LT activation of caspase-1 is known to require the inflammasome adapter Nalp1. In contrast, Salmonella infection activated caspase-1 through an independent pathway requiring the inflammasome adapter Ipaf. These distinct mechanisms of caspase-1 activation converged on a common pathway of caspase-1-dependent cell death featuring DNA cleavage, cytokine activation, and, ultimately, cell lysis resulting from the formation of membrane pores between 1.1 and 2.4 nm in diameter and pathological ion fluxes that can be blocked by glycine. These findings demonstrate that distinct activation pathways elicit the conserved cell death effector mechanism of caspase-1-mediated pyroptosis and support the notion that this pathway of proinflammatory programmed cell death is broadly relevant to cell death and inflammation invoked by diverse stimuli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


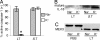
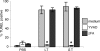
References
-
- Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. - PubMed
-
- Wang W, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol. 2005;288:F997–F1004. - PubMed
-
- Frantz S, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

