Distinct effects of the recurrent Mlh1G67R mutation on MMR functions, cancer, and meiosis
- PMID: 18337503
- PMCID: PMC2393764
- DOI: 10.1073/pnas.0800276105
Distinct effects of the recurrent Mlh1G67R mutation on MMR functions, cancer, and meiosis
Abstract
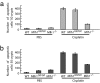
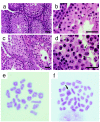
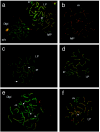
Mutations in the human DNA mismatch repair (MMR) gene MLH1 are associated with hereditary nonpolyposis colorectal cancer (Lynch syndrome, HNPCC) and a significant proportion of sporadic colorectal cancer. The inactivation of MLH1 results in the accumulation of somatic mutations in the genome of tumor cells and resistance to the genotoxic effects of a variety of DNA damaging agents. To study the effect of MLH1 missense mutations on cancer susceptibility, we generated a mouse line carrying the recurrent Mlh1(G67R) mutation that is located in one of the ATP-binding domains of Mlh1. Although the Mlh1(G67R) mutation resulted in DNA repair deficiency in homozygous mutant mice, it did not affect the MMR-mediated cellular response to DNA damage, including the apoptotic response of epithelial cells in the intestinal mucosa to cisplatin, which was defective in Mlh1(-/-) mice but remained normal in Mlh1(G67R/G67R) mice. Similar to Mlh1(-/-) mice, Mlh1(G67R/G67R) mutant mice displayed a strong cancer predisposition phenotype. However, in contrast to Mlh1(-/-) mice, Mlh1(G67R/G67R) mutant mice developed significantly fewer intestinal tumors, indicating that Mlh1 missense mutations can affect MMR tumor suppressor functions in a tissue-specific manner. In addition, Mlh1(G67R/G67R) mice were sterile because of the inability of the mutant Mlh1(G67R) protein to interact with meiotic chromosomes at pachynema, demonstrating that the ATPase activity of Mlh1 is essential for fertility in mammals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

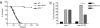


References
-
- Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: Database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997;113:1146–1158. - PubMed
-
- Yan H, et al. Conversion of diploidy to haploidy. Nature. 2000;403:723–724. - PubMed
-
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. - PubMed
-
- Schofield MJ, Hsieh P. DNA mismatch repair: Molecular mechanisms and biological function. Annu Rev Microbiol. 2003;57:579–608. - PubMed
-
- Kolas NK, Cohen PE. Novel and diverse functions of the DNA mismatch repair family in mammalian meiosis and recombination. Cytogenet Genome Res. 2004;107:216–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA13330/CA/NCI NIH HHS/United States
- CA84301/CA/NCI NIH HHS/United States
- R01 CA076329/CA/NCI NIH HHS/United States
- R01 CA102705/CA/NCI NIH HHS/United States
- R56 HD041012/HD/NICHD NIH HHS/United States
- CA102705/CA/NCI NIH HHS/United States
- CA93484/CA/NCI NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- CA76329/CA/NCI NIH HHS/United States
- R01 HD041012/HD/NICHD NIH HHS/United States
- U01 CA084301/CA/NCI NIH HHS/United States
- R01 CA093484/CA/NCI NIH HHS/United States
- HD41012/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

