TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype
- PMID: 18337563
- PMCID: PMC2384130
- DOI: 10.1182/blood-2007-08-108597
TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype
Abstract
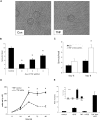
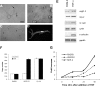
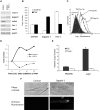
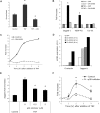
Pathological angiogenesis associated with wound healing often occurs subsequent to an inflammatory response that includes the secretion of cytokines such as tumor necrosis factor (TNF). Controversy exists on the angiogenic actions of TNF, with it being generally proangiogenic in vivo, but antiangiogenic in vitro. We find that whereas continuous administration of TNF in vitro or in vivo inhibits angiogenic sprouting, a 2- to 3-day pulse stimulates angiogenesis by inducing an endothelial "tip cell" phenotype. TNF induces the known tip cell genes platelet-derived growth factor B (PDGFB) and vascular endothelial cell growth factor receptor-2 (VEGFR2), while at the same time blocking signaling through VEGFR2, thus delaying the VEGF-driven angiogenic response. Notch signaling regulates tip cell function, and we find that TNF also induces the notch ligand jagged-1, through an NFkappaB-dependent mechanism. Enrichment of jagged-1 in tip cells was confirmed by immunofluorescent staining as well as by laser capture microdissection/quantitative reverse-transcription-polymerase chain reaction (qRT-PCR) of tip cells sprouting in vitro. Thus, in angiogenesis, the temporal expression of TNF is critical: it delays angiogenesis initially by blocking signaling through VEGFR2, but in addition by inducing a tip cell phenotype through an NFkappaB-dependent pathway, it concomitantly primes endothelial cells (ECs) for sprouting once the initial inflammatory wave has passed.
Figures


References
-
- Lokmic Z, Darby IA, Thompson EW, Mitchell GM. Time course analysis of hypoxia, granulation tissue and blood vessel growth, and remodeling in healing rat cutaneous incisional primary intention wounds. Wound Repair Regen. 2006;14:277–288. - PubMed
-
- Mano-Hirano Y, Sato N, Sawasaki Y, et al. Inhibition of tumor-induced migration of bovine capillary endothelial cells by mouse and rabbit tumor necrosis factor. J Natl Cancer Inst. 1987;78:115–120. - PubMed
-
- Sato N, Goto T, Haranaka K, et al. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst. 1986;76:1113–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

